Abstract
In order to improve biosurfactant production by Yarrowia lipolytica IMUFRJ 50682, a factorial design was carried out. A 24 full factorial design was used to investigate the effects of nitrogen sources (urea, ammonium sulfate, yeast extract, and peptone) on maximum variation of surface tension (ΔST) and emulsification index (EI). The best results (67.7% of EI and 20.9 mN m−1 of ΔST) were obtained in a medium composed of 10 g 1−1 of ammonium sulfate and 0.5 g 1−1 of yeast extract. Then, the effects of carbon sources (glycerol, hexadecane, olive oil, and glucose) were evaluated. The most favorable medium for biosurfactant production was composed of both glucose (4% w/v) and glycerol (2% w/v), which provided an EI of 81.3% and a ΔST of 19.5 mN m−1. The experimental design optimization enhanced ΔEI by 110.7% and ΔST by 108.1% in relation to the standard process.
1. Introduction
Biological surfactants are molecules that can be produced extracellulary or as a part of the cell membrane by yeast, bacteria and fungi [1]. They are amphiphilic compounds which can reduce surface and interfacial tensions in both aqueous solutions and hydrocarbon mixtures [2]. Biosurfactants are often produced in media containing n-alkanes or other water-immiscible substrates in order to facilitate cell adhesion to hydrophobic substrates. However, some microbial surfactants can be produced on water-soluble substrates [3, 4].
Biosurfactants can be as effective as synthetic surfactants and, for certain applications, present some advantages such as high specificity and biodegradability [5]. In recent years interest in biosurfactants has considerably increased, as these molecules are potential candidates for many commercial applications in the petroleum, pharmaceutical, biomedical and food industrial processes.
Nowadays, the use of biosurfactants has been limited due to the high production cost. Thus, the production medium optimization is one of the key factors. In researchers that have studied the medium composition influence on biosurfactant production, the parameters that mostly affected the economics of biosurfactant manufacture include the choice of nutrients and yeast strain [6].
Yarrowia lipolytica, strictly aerobic yeast, exhibits a diverse range of metabolic activities. It is considered nonpathogenic and several processes using this organism were classified as generally recognized as safe (GRAS) by the Food and Drug Administration (FDA, USA) [7]. Some species have the degradation ability for a variety of organic compounds, including aliphatic and aromatic hydrocarbons and this property is often accompanied by biosurfactants production ability [8]. These molecules are predominantly glycolipids, but other types have also been reported using different substrates [4, 9].
Amaral et al. [3] isolated a bioemulsifier, named Yansan, from a glucose-based culture medium of Y. lipolytica IMUFRJ 50682, in the absence of any water-immiscible substrate. The aim of the present work was to improve the standard medium used by Amaral et al. [3] for a biosurfactant production using a sequence of experimental design and surface response method. The influence of system aeration, agitation speed and carbon and nitrogen sources were evaluated.
2. Materials and Methods
2.1. Microorganism and Culture Conditions
A wild type strain of Yarrowia lipolytica (IMUFRJ 50682) was employed [10] and kept at 4°C on YPD-agar medium. For inoculum conditions, cells were cultivated at 28°C in a rotary shaker at 160 rpm, in 500 mL shake flasks containing 200 mL of YPD medium (%w/v: yeast extract (Oxoid), (1); peptone (Oxoid), 0.64; glucose (Reagen), (2). After 48 hours of cultivation, these cells were used in sufficient amount to inoculate 1 mg of cells (dry weight) per mL of biosurfactant production media. All biosurfactant production experiments were carried out in 1000 mL shake flasks in a rotary shaker at 28°C. The agitation speed, the medium volume and its composition are specified along the text.
2.2. Effect of Aeration and Agitation Speed
Different medium volumes (300 and 500 mL) were used in 1000 mL shake flasks in a rotary shaker at 160 and 250 rpm at 28°C. In this study, YPD medium was used for biosurfactant production.
2.3. Nitrogen Source Evaluation
A 24 full factorial design was carried out to verify the effects and interactions of urea (Vetec), ammonium sulfate (Vetec), yeast extract (Oxoid), and peptone (Oxoid). “STATISTICA” (version 7.0) software was used for regression and graphical analyses of the data obtained. In this design, a set of 19 experiments, including three replicates at the central point, was performed. The range and the levels of the variables herein investigated are given in Table 1. The maximum variation of surface tension and emulsification index were taken as dependent variables of the experimental design. The components of each medium were dissolved in 500 mL of distilled water, with 2% w/v of glucose as carbon source.
Table 1.
Experimental range and levels of the independent variables used in the 24 full factorial design for the nitrogen source study.
| Variable (g l−1) | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Peptone (x1) | 0 | 6.4 | 12.8 |
| Yeast extract (x2) | 5 | 10 | 15 |
| Ammonium sulfate (x3) | 0 | 5 | 10 |
| Urea (x4) | 0 | 0.1 | 0.2 |
2.4. Carbon Source Optimization
The carbon sources used in biosurfactant production experiments were glycerol, hexadecane, olive oil and glucose. In order to identify which carbon source effects significantly biosurfactant production, a 24 full factorial design was carried. Similarly to the nitrogen source study, a set of 19 experiments with tree replicates at the central point was performed. The range and levels of the variables investigated are given in Table 2. The maximum variation in surface tension and emulsification index were also taken as dependent variables of the designed experiments.
Table 2.
Experimental range and levels of the independent variables used in the 24 full factorial design for the carbon source study.
| Variable (% w/v) | Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Glycerol (z1) | 0 | 1 | 2 |
| Olive oil (z2) | 0 | 2 | 4 |
| Hexadecane (z3) | 0 | 1 | 2 |
| Glucose (z4) | 0 | 2 | 4 |
2.5. Analytical Methods
Along biosurfactant production experiments, samples were taken every 24 hours for the determination of: surface tension (ST), emulsification index (EI), oil spreading (OS), cell growth and glucose concentration. Besides cell growth, all other methods were performed in cell-free broth, obtained by sample centrifugation at 1000 g for 10 minutes.
2.5.1. Surface Tension (ST)
The surface tension was determined on cell-free broth with a Tensiometer K 100 (Kruss) using the ring method at room temperature (25 ± 2°C).
2.5.2. Emulsification Index (EI)
The emulsification index was determined by using a modification of the method described by Iqbal et al. [11]. The EI of cell-free samples was determined by adding 1 mL of hexadecane to the same amount of sample, vortex-mixing for 2 minutes and leaving to stand for 24 hours. The EI is given as a percentage of emulsified layer height (cm) divided by total height of the liquid column (cm).
2.5.3. Oil Spreading Technique (OS)
The oil spreading technique was adapted from the method described by Youssef et al. [12]. Fifty milliliters of distilled water were added to a Petri dish followed by addition of 40 μl of crude oil to the water surface. Fifteen microliters of cell-free samples were then added to the oil surface. The diameter of the clear zone formed on the oil surface was measured with caliper rule.
2.5.4. Cell Growth Determination
Cell concentration was followed by optical density measurements at 570 nm and the OD values were converted to cell dry weight per volume (mg dw/mL) using a factor previously determined [3].
2.5.5. Glucose Concentration
Glucose was determined by enzymatic analysis (glucose oxidase method), with a ready-to-use diagnostic unit (HUMAN GmbH Germany).
3. Results
3.1. Influence of Aeration and Agitation Speed
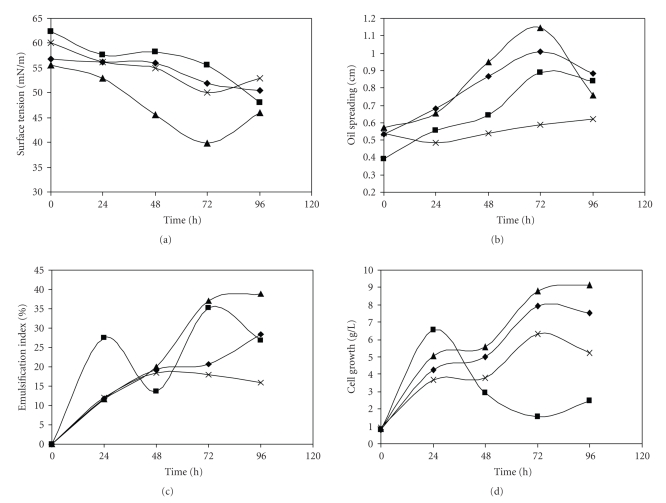
Biosurfactant production by Y. lipolytica was influenced by system aeration and agitation speed. The results from batch fermentation show that as the agitation speed increases from 160 rpm to 250 rpm, biosurfactant production increases as determined through the tree different methods used to measure biosurfactant activity (Figure 1).
Figure 1.
Kinetics of biosurfactant production by Yarrowia lipolytica: surface tension (a), oil spreading technique (b), emulsification index (c) and cell growth (d). Vm/Vf 0.3 and 160 rpm ( ); Vm/Vf 0.3 and 250 rpm (
); Vm/Vf 0.3 and 250 rpm ( ); Vm/Vf 0.5 and 250 rpm (
); Vm/Vf 0.5 and 250 rpm ( ) and Vm/Vf 0.5 and 160 rpm.
) and Vm/Vf 0.5 and 160 rpm.
The influence of aeration and agitation speed was investigated in experiments carried under several combinations of Vm/Vf values (ratio between medium volume and flask volume) and agitation speeds. The results presented in Figure 1 show that the increase of Vm/Vf ratio from 0.3 to 0.5 raised biosurfactant production. The sudden reduction in cell growth concentration after 24 hours for Vm/Vf of 0.5 and 250 rpm (Figure 1(d)) was due to the migration of cells to the formed foam. Since cell concentration was measured in the aqueous phase and a significant portion of cells migrates to the foam as the agitation starts, a consequence is a reduction in cell concentration in the aqueous medium.
At this moment, the most efficient biosurfactant production was achieved at 250 rpm and a Vm/Vf ratio of 0.5. The values obtained for biosurfactant activity in this condition were; 14.3 mN m−1 of ΔST, 38.9% of EI and 1.1 cm of OS. Therefore, this condition was used for the subsequent experiments.
3.2. Optimization of Nitrogen Source
The factorial design enables the identification of the nitrogen sources that play a significant role on biosurfactant production. Table 3 presents the results of the 24 experimental design performed to achieve the nitrogen source optimization. Data presented in Table 3 indicates that ΔST and EI vary markedly from 4.0 to 21.1 mN m−1 and from 1.0 to 60.4%, respectively.
Table 3.
Experimental design and results of the 24 full factorial design for nitrogen source evaluation.
| Run | x1* | x2* | x3* | x4* | ST† | EI‡ |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 6.5 | 37.3 |
| 2 | 1 | −1 | −1 | −1 | 14.5 | 26.1 |
| 3 | −1 | 1 | −1 | −1 | 5.1 | 27.0 |
| 4 | 1 | 1 | −1 | −1 | 4.0 | 1.0 |
| 5 | −1 | −1 | 1 | −1 | 16.6 | 45.3 |
| 6 | 1 | −1 | 1 | −1 | 22.0 | 52.2 |
| 7 | −1 | 1 | 1 | −1 | 19.5 | 40.6 |
| 8 | 1 | 1 | 1 | −1 | 13.0 | 50.0 |
| 9 | −1 | −1 | −1 | 1 | 7.5 | 35.7 |
| 10 | 1 | −1 | −1 | 1 | 6.0 | 30.6 |
| 11 | −1 | 1 | −1 | 1 | 9.3 | 13.0 |
| 12 | 1 | 1 | −1 | 1 | 4.6 | 6.2 |
| 13 | −1 | −1 | 1 | 1 | 15.2 | 40.3 |
| 14 | 1 | −1 | 1 | 1 | 16.3 | 50.1 |
| 15 | −1 | 1 | 1 | 1 | 21.1 | 60.4 |
| 16 | 1 | 1 | 1 | 1 | 9.4 | 43.2 |
| 17 | 0 | 0 | 0 | 0 | 13.0 | 26.0 |
| 18 | 0 | 0 | 0 | 0 | 11.4 | 24.0 |
| 19 | 0 | 0 | 0 | 0 | 11.0 | 25.4 |
*The coded variables xi (i = 1, 2, 3, 4) are defined in Table 1. †Variation in surface tension (mN m−1), ‡Emulsification index (%).
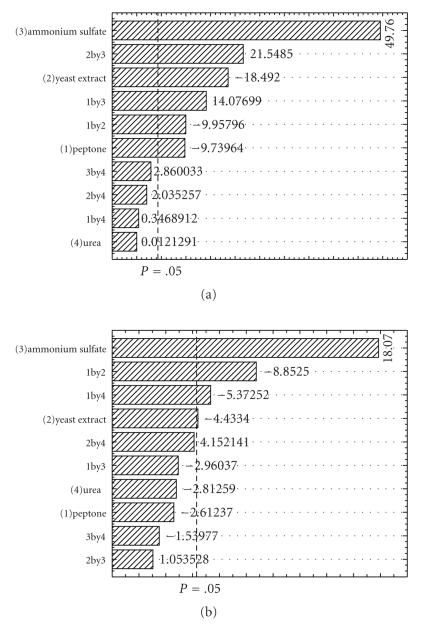
Figure 2 illustrates the Pareto chart, with 95% of confidence level, for the estimated effects in absolute values for ΔST and EI. It is possible to observe that ammonium sulfate and yeast extract had significantly influenced both dependent variables, ΔST and EI. On the other hand, urea did not significantly influence ΔST nor EI in the range studied. These results are in accordance with the literature [8, 13, 14]. Peptone was only statistically significant for EI. Figure 2 also depicts that the increase in ammonium sulfate concentration and the interaction of ammonium sulfate and yeast extract showed positive effects on ΔST and EI. However, the increase in yeast extract produced a negative effect.
Figure 2.
Pareto Chart of standardized effects for emulsification index (a) and Δ surface tension (b) for the 24 full factorial design used in the optimization of nitrogen source. The point at which the effects estimates were statistically significant (at P = .05) is indicated by the broken vertical line.
The dependence of variables ΔST and EI within nitrogen sources could be written as shown by (1):
| (1) |
The variance analysis of the first order model for ΔST and EI shows that the model is highly significant, as is evident from the fisher F test, where the calculated F values (FST = 25.4; FEI = 10.2) are greater than the tabular F value (F0.05;4;14 = 3.1, F0,05;6;12 = 3.0). The values for the determined coefficients were 0.87 for ΔST and 0.86 for EI. The best ΔST and EI values (21.1 mN m−1 and 60.4%) were obtained with 10 g l−1 of ammonium sulfate and 5 g l−1 of yeast extract.
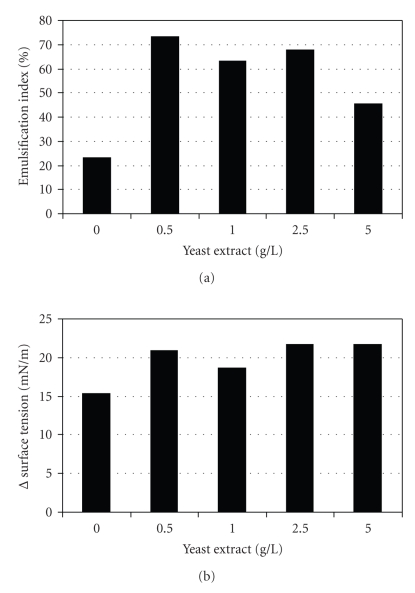
In order to investigate the negative effect of yeast extract four experiments were carried out with different yeast extract concentrations (0.5; 1; 2.5; 5 g l−1). The ammonium sulfate concentration in the medium was kept constant at 10g l−1. The results show that 0.5 g l−1 of yeast extract was the best condition, reaching 20.9 mN m−1 for ΔST and 73.1% for EI (Figure 3).
Figure 3.
Biosurfactant production by Yarrowia lipolytica with different YE concentration. (a) Emulsification index and (b) maximum variation of surface tension.
3.3. Optimization of Carbon Source
Carbon source type also plays a critical role in the performance of biosurfactant production by microorganisms [4, 15, 16]. In the present study both hydrophobic and hydrophilic carbon sources were evaluated for the biosurfactant production by a 24 full factorial design.
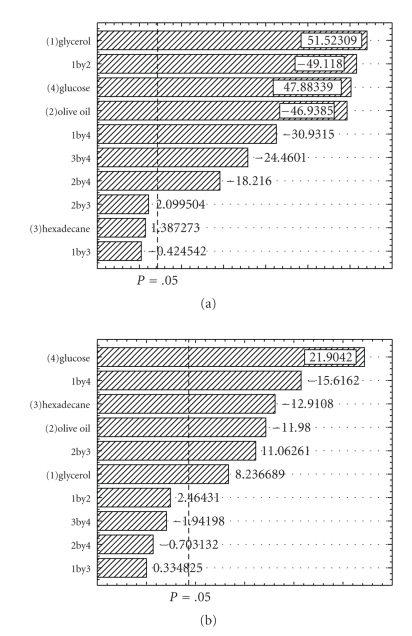
The experimental design and results are presented in Table 4. The data indicates that high EI (82.9%) and ΔST (27.8 mN m−1) values were obtained when glucose and glycerol concentrations were high. Figure 4 shows the Pareto chart, where is possible to identify that, for EI and ΔST, glucose and glycerol concentrations had a positive significant effect. Although the effect of olive oil is statistically significant for both methods, it presents a negative effect, that is, increasing its concentration, the surfactant production diminishes. Identical response was obtained with hexadecane, however it haven't statistically influence on ΔST.
Table 4.
Experimental design and results of the 24 full factorial design for carbon source analyses.
| Run | z1* | z2* | z3* | z4* | ST† | EI‡ |
|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | −1 | 9.0 | 6.9 |
| 2 | 1 | −1 | −1 | −1 | 19.5 | 62.3 |
| 3 | −1 | 1 | −1 | −1 | 2.0 | 11.6 |
| 4 | 1 | 1 | −1 | −1 | 11.0 | 25.5 |
| 5 | −1 | −1 | 1 | −1 | 2.6 | 12.8 |
| 6 | 1 | −1 | 1 | −1 | 10.7 | 70.2 |
| 7 | −1 | 1 | 1 | −1 | 2.0 | 27.5 |
| 8 | 1 | 1 | 1 | −1 | 9.9 | 39.3 |
| 9 | −1 | −1 | −1 | 1 | 27.8 | 56.2 |
| 10 | 1 | −1 | −1 | 1 | 20.2 | 82.9 |
| 11 | −1 | 1 | −1 | 1 | 14.4 | 48.8 |
| 12 | 1 | 1 | −1 | 1 | 14.8 | 40.0 |
| 13 | −1 | −1 | 1 | 1 | 14.9 | 47.3 |
| 14 | 1 | −1 | 1 | 1 | 12.6 | 76.8 |
| 15 | −1 | 1 | 1 | 1 | 14.4 | 38.9 |
| 16 | 1 | 1 | 1 | 1 | 13.0 | 26.0 |
| 17 | 0 | 0 | 0 | 0 | 10.9 | 42.3 |
| 18 | 0 | 0 | 0 | 0 | 9.8 | 43.1 |
| 19 | 0 | 0 | 0 | 0 | 11.2 | 43.9 |
*The coded variables zi (i = 1, 2, 3, 4) are defined in Table 2. †Variation in surface tension (mN m−1), ‡Emulsification index (%).
Figure 4.
Pareto Chart of standardized effects for emulsification index (a) and Δ surface tension (b) for the 24 full factorial design used in the optimization of carbon source.
Thus, glycerol and glucose were the best substrates to increase biosurfactant production, allowing its release in the medium, with an EI of 82.9% and a ΔST of 27.5 mN m−1.
In order to analyze the influence of the interaction between glycerol and glucose and the carbon to nitrogen ratio (C/N) in the biosurfactant production, a second experimental design was carried out with a 22 central composite design. The concentrations of the nitrogen source, ammonium sulfate and yeast extract, remained 10 g l−1 and 0.5 g l−1, respectively. The concentration ranges of glucose and glycerol concentrations, indicated in Table 5, were calculated according to the results of the previous factorial design. The results for the two-factorial central composite design are present in Table 6. High EI and ΔST values were found at central level conditions (zero level, run no. 9, 10 and 11). The average EI and ΔST at zero level were 81.3% and 19.5 mN m−1, 2.1 and 2.0 fold, respectively, higher than the standard biosurfactant production process (38.1% and 9.6 m Nm−1).
Table 5.
Coded and actual levels of the two variables in the experimental design.
| Variable | Level | ||||
|---|---|---|---|---|---|
| −1.41 | −1 | 0 | 1 | +1.41 | |
| Glycerol (z1) (% v.v−1) | 0.59 | 1 | 2 | 3 | 3.41 |
| Glucose (z4) (% w. v−1) | 1.17 | 2 | 4 | 6 | 6.83 |
Table 6.
Experimental design and results of the central composite design.
| Run | z1a | z4a | C/N | EIb | STc |
|---|---|---|---|---|---|
| 1 | −1 | −1 | 5.9 | 51.4 | 11.8 |
| 2 | +1 | −1 | 10.4 | 66.2 | 13.6 |
| 3 | −1 | +1 | 13.3 | 60.8 | 12.9 |
| 4 | +1 | +1 | 17.8 | 67.3 | 16.0 |
| 5 | −1.41 | 0 | 8.7 | 54.1 | 12.1 |
| 6 | +1.41 | 0 | 15.1 | 57.8 | 15 |
| 7 | 0 | −1.41 | 6.6 | 61.4 | 12.4 |
| 8 | 0 | +1.41 | 17.1 | 73.7 | 16.2 |
| 9 | 0 | 0 | 11.9 | 81.8 | 19.5 |
| 10 | 0 | 0 | 11.9 | 81.1 | 19.0 |
| 11 | 0 | 0 | 11.9 | 80.9 | 20.1 |
aThe coded variables zi (i = 1, 4) are defined in Table 5. bEmulsification index (%) Variation in surface tension (mN m−1)c.
The effect of carbon to nitrogen ratio (C/N) was also analyzed in this experimental design. As indicated in Table 6, the C/N was 11.9 in the central point, where the best biosurfactant production occurred. In contrast, when the C/N was lower or higher, the biosurfactant production was not favored.
According to the response values obtained from the designed experiments (Table 6), a second-order regression equation was calculated for the response surface as follows:
| (2) |
The model was checked and it was found to be adequate as expressed by the coefficient of determination (R2), which was calculated to be 0.99 for both EI and ΔST. The variance analysis of the quadratic model for ΔST and EI shows that the model is highly significant, as is evident from the fisher F test, where the calculated F values, FST = 303.2 and FEI = 179.7, are greater than the tabular F values, F0.05;4;6 = 4.5 and F0.05;3,4 = 6.5.
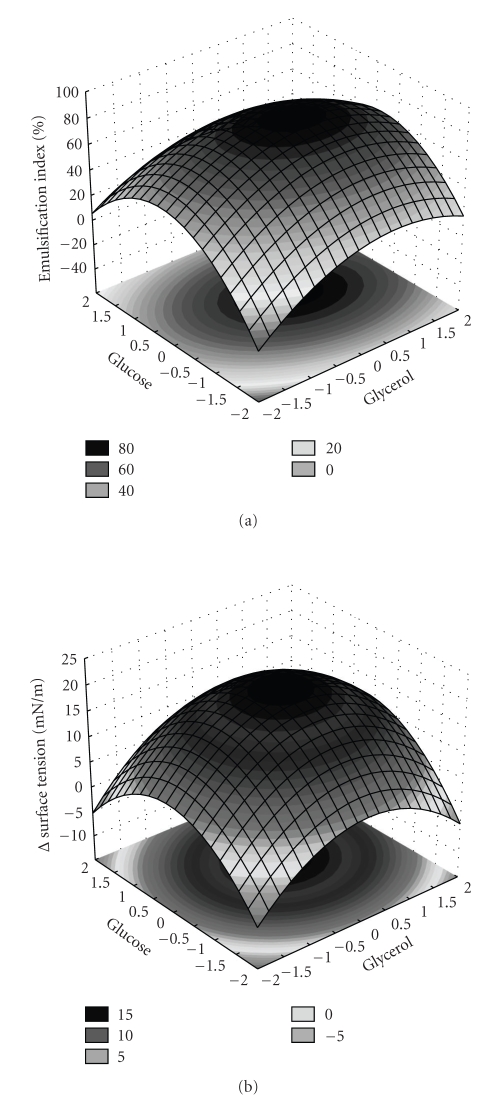
Based on the model equation, the surface responses were plotted as shown in Figure 5, where is possible to observe that the optimal response occurred near the central point of glucose and glycerol concentrations for both methods EI and ΔST. The validation of the mathematical model was performed using the mean values achieved in the central points. The experimental maximum EI and ΔST perfectly agreed with predicted maximum. The difference between the model prediction and the experimental data was less than 0.5%.
Figure 5.
Three-dimensional response surface showing the effect of glucose and glycerol on variation of emulsification index (a) and surface tension (b).
4. Discussion
Statistical optimization of medium components for biosurfactant production by Y. lipolytica was performed using experimental design and surface response methodologies. System agitation and aeration conditions were first established and the best condition was 250 rpm and Vm/Vf of 0.5, achieving 38.9% of EI and 9.6 mN m−1 of ΔST. This is consistent with the results described by Yeh et al. [17] and Amaral et al. [3] who have mentioned that biosurfactant production rose with the increase of agitation speed. Kronemberger et al. [18] have shown that a rhamnolipid production by Pseudomonas aeruginosa depend on the specific oxygen uptake rate. The agitation speed affects the mass transfer efficiency of both oxygen molecules and medium components. These parameters are considered crucial to cell growth and biosurfactant formation by the strictly aerobic yeast Y. lipolytica.
Highest emulsification activity values detected in shake flasks with higher agitation speed can be linked to the physiological function of the biosurfactants. It has been suggested that biosurfactant production can increase the solubility of hydrophobic substrates in water and, consequently, facilitate the transport of nutrients to microorganisms. Therefore, a larger shear stress can induce larger biosurfactant excretion since the contact between the organic phase drops, dispersed in water, and the microorganisms becomes more difficult. The opposite may happen with other microorganisms: an increase in agitation speed can result in a reduction of biosurfactant yield due to the shear effect, causing cells mechanical stress [19].
In the case of yeasts, as Y. lipolytica, an increase in the agitation speed possibly favors the liberation of the surfactants attached to the cell wall increasing the amount of free surfactant in the culture medium. Desai and Banat [2], in their review, mentioned that in the case of yeast, biosurfactant production usually increases in higher agitation speed and aeration, corroborating with the results accomplished in this work.
Maximum biosurfactant production was found in Vm/Vf ratio of 0.5. This can be attributed to the severe foaming when the flask with Vm/Vf ratio of 0.3 was shaken at 250 rpm. The reduction in Vm/Vf ratio modifies significantly the medium oxygenation because it increases the gas-liquid interfacial area and promotes foam formation. The heavy foaming may decrease the oxygen transfer efficiency and might also remove cells and biosurfactant molecules from the liquid medium, decreasing the yield of these metabolites [20].
Different nitrogen sources were evaluated on biosurfactant production by Y. lipolytica. In the production medium, a nitrogen source is needed for cell growth, with great importance for proteins and enzymes synthesis. Based on the results, ammonium sulfate and yeast extract demonstrate to be the best nitrogen sources for cell growth and biosurfactant synthesis. When yeast extract concentration is low, biosurfactant production is favored. This fact was also observed by Casas and Garcia-Ochoa [21]. They pointed out that when nitrogen source is in excess biosurfactant production decreases because carbon source is used for yeast growth. Kim et al. [22] compared organic and inorganic nitrogen sources for biosurfactant production by Candida Antarctica. The biosurfactant synthesis was repressed, in spite of abundant cells and high cell growth rate, when organic nitrogen was used instead of inorganic one because it was preferentially utilized for cell growth rather than biosurfatant production. In literature, several works show the influence of this nutrient on biosurfactant formation and yeast extract is the most frequently used, but its optimal concentration is not clear. While Copper and Paddock [14] found 5 g L−1 as an optimal concentration, Zhou and kosaric [15] obtained higher biosurfactant concentration with approximately 2.5 g L−1.
Among the carbon substrates herein evaluated, glucose and glycerol were the most efficient ones for biosurfactant production by Y. lipolytica. The results show that when Y. lipolytica was cultivated with a hydrophobic substrate as carbon source (hexadecane and olive oil) the biosurfactant production is not favored. This can be attributed to the association of surfactants with Y. lipolytica's cell wall. Several works have reported this phenomenon. On the other hand, the use of glycerol as carbon source allowed the release of a biosurfactant produced by Rhodococcus erythropolis, originally associated to the cell wall as reported by Ciapina et al. [16]. According to Lang and Philp [23] only a minor portion of the produced surfactants is released when a hydrophobic substrate is used.
Physiologically, biosurfactant production is associated with the assimilatory mechanism of hydrophobic substrates. This mechanism would consist in direct contact of cells with large oil droplets, with little or no emulsification, or the contact with fine oil droplets, culminating in emulsification. In the first, the biosurfactant is retained on the outer cell surface, facilitating the attachment and subsequent transport of hydrophobic compounds into the cell [16]. In the second case, the free biosurfactant, released in the culture medium, would form a hydrocarbon-surfactant complex that pseudo-solubilize the substrates and, hence, increase availability to the cell [24].
Other phenomenon observed during the experiments with hexadecane and olive oil as carbon source was cells migration to the organic phase and a reduction in cell concentration in the aqueous medium. Amaral et al. [3] investigated the surface characteristics of Y. lipolytica and showed that it has a hydrophobic character and high cell adhesion to non-polar solvents. Thus, glycerol and glucose were the best substrates to increase biosurfactant production by Y. lipolytica, allowing its release in the medium.
The best C/N ratio for biosurfactant production was 11.9. Fonseca et al. [25] reported an inferior value of C/N (3.0) for a biosurfactant produced by Bacillus subtilis.
The experimental design optimization enhanced EI and ΔST of the standard biosurfactant process by 110.7% and 108.1%, respectively. It demonstrates that the response surface method (RSM) is an effective tool for the improvement of medium composition leading to a higher biosurfactant production. Using RSM analyses, optimal concentrations for glucose (4% w/v), glycerol (2% w/v), ammonium sulfate (10 g l−1) and yeast extract (0.5 g l−1) were identified, for the production of an EI of 81.3% and a ΔST of 19.5 mN m−1. Comparing with Ciapina et al. [16], the biosurfactant produced in the present work presents a low ΔST and a high EI, showing that the molecule presents an emulsifier characteristic.
Acknowledgments
The authors kindly acknowledge the financial aid and research scholarships given by Conselho Nacional de desenvolvimento Científico e Tecnológico (CNPq) and Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ).
Nomenclature
- C/N:
Carbon to nitrogen ratio
- EI:
Emulsification index
- OS:
Oil spreading technique
- RSM:
Surface response methodologies
- ΔST:
Variation of surface tension
- Vm/Vf:
Media Volume/Flask volume.
References
- 1.Mulligan CN. Environmental applications for biosurfactants. Environmental Pollution. 2005;133(2):183–198. doi: 10.1016/j.envpol.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 2.Desai JD, Banat IM. Microbial production of surfactants and their commercial potential. Microbiology and Molecular Biology Reviews. 1997;61(1):47–64. doi: 10.1128/mmbr.61.1.47-64.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amaral PFF, da Silva JM, Lehocky M, et al. Production and characterization of a bioemulsifier from Yarrowia lipolytica. Process Biochemistry. 2006;41(8):1894–1898. [Google Scholar]
- 4.Sarubbo LA, Marçal MDC, Neves MLC, Silva MDPC, Porto LF, Campos-Takaki GM. Bioemulsifier production in batch culture using glucose as carbon source by Candida lipolytica. Applied Biochemistry and Biotechnology. 2001;95(1):59–67. doi: 10.1385/abab:95:1:59. [DOI] [PubMed] [Google Scholar]
- 5.Gautam KK, Tyagi VK. A review of microbial surfactant. Journal of Oleo Science. 2006;55:155–166. [Google Scholar]
- 6.Fiechter A. Biosurfactants: moving towards industrial application. Trends in Biotechnology. 1992;10(6):208–217. doi: 10.1016/0167-7799(92)90215-h. [DOI] [PubMed] [Google Scholar]
- 7.Barth G, Gaillardin C. Physiology and genetics of the dimorphic fungus Yarrowia lipolytica. FEMS Microbiology Reviews. 1997;19(4):219–237. doi: 10.1111/j.1574-6976.1997.tb00299.x. [DOI] [PubMed] [Google Scholar]
- 8.Albuquerque CDC, Filetti AMF, Campos-Takaki GM. Optimizing the medium components in bioemulsifiers production by Candida lipolytica with response surface method. Canadian Journal of Microbiology. 2006;52(6):575–583. doi: 10.1139/w06-002. [DOI] [PubMed] [Google Scholar]
- 9.Cirigliano MC, Carman GM. Isolation of a bioemulsifier from Candida lipolytica. Applied and Environmental Microbiology. 1984;48(4):747–750. doi: 10.1128/aem.48.4.747-750.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haegler AN, Mendonça-Haegler LC. Yeasts from marine and estuarine waters with different levels of pollution in the State of Rio de Janeiro, Brazil. Applied and Environmental Microbiology. 1981;41:173–178. doi: 10.1128/aem.41.1.173-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal S, Khalid ZM, Malik KA. Enhanced biodegradation and emulsification of crude oil and hyperproduction of biosurfactants by a gamma ray-induced mutant of Pseudomonas aeruginosa. Letters in Applied Microbiology. 1995;21(3):176–179. doi: 10.1111/j.1472-765x.1995.tb01035.x. [DOI] [PubMed] [Google Scholar]
- 12.Youssef NH, Duncan KE, Nagle DP, Savage KN, Knapp RM, McInerney MJ. Comparison of methods to detect biosurfactant production by diverse microorganisms. Journal of Microbiological Methods. 2004;56(3):339–347. doi: 10.1016/j.mimet.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Zinjarde SS, Pant A. Emulsifier from a tropical marine yeast, Yarrowia lipolytica NCIM 3589. Journal of Basic Microbiology. 2002;42(1):67–73. doi: 10.1002/1521-4028(200203)42:1<67::AID-JOBM67>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 14.Cooper DG, Paddock DA. Torulopsis petrophilum and surface activity. Applied and Environmental Microbiology. 1983;46(6):1426–1429. doi: 10.1128/aem.46.6.1426-1429.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou QH, Kosaric N. Utilization of canola oil and lactose to produce biosurfactant with Candida bombicola. Journal of the American Oil Chemists’ Society. 1995;72:89–91. [Google Scholar]
- 16.Ciapina EMP, Melo WC, Santa Anna LMM, Santos AS, Freire DMG, Pereira N., Jr. Biosurfactant production by Rhodococcus erythropolis grown on glycerol as sole carbon source. Applied Biochemistry and Biotechnology. 2006;131(1–3):880–886. doi: 10.1385/ABAB:131:1:880. [DOI] [PubMed] [Google Scholar]
- 17.Yeh M-S, Wei Y-H, Chang J-S. Bioreactor design for enhanced carrier-assisted surfactin production with Bacillus subtilis. Process Biochemistry. 2006;41(8):1799–1805. [Google Scholar]
- 18.Kronemberger FDA, Santa Anna LMM, Fernandes ACLB, Menezes RRD, Borges CP, Freire DMG. Oxygen-controlled biosurfactant production in a bench scale bioreactor. Applied Biochemistry and Biotechnology. 2008;147(1–3):33–45. doi: 10.1007/s12010-007-8057-3. [DOI] [PubMed] [Google Scholar]
- 19.Moussa TAA, Ahmed GM, Abdel-hamid SM-S. Optimization of cultural conditions for biosurfactant production from Nocardia amarae. Journal of Applied Sciences Research. 2006;2:844–850. [Google Scholar]
- 20.Wei Y-H, Chou C-L, Chang J-S. Rhamnolipid production by indigenous Pseudomonas aeruginosa J4 originating from petrochemical wastewater. Biochemical Engineering Journal. 2005;27(2):146–154. [Google Scholar]
- 21.Casas JA, García-Ochoa F. Sophorolipid production by Candida bombicola: medium composition and culture methods. Journal of Bioscience and Bioengineering. 1999;88(5):488–494. doi: 10.1016/s1389-1723(00)87664-1. [DOI] [PubMed] [Google Scholar]
- 22.Kim H-S, Jeon J-W, Kim B-H, Ahn C-Y, Oh H-M, Yoon B-D. Extracellular production of a glycolipid biosurfactant, mannosylerythritol lipid, by Candida sp. SY16 using fed-batch fermentation. Applied Microbiology and Biotechnology. 2006;70(4):391–396. doi: 10.1007/s00253-005-0092-9. [DOI] [PubMed] [Google Scholar]
- 23.Lang S, Philp JC. Surface-active lipids in rhodococci. Antonie van Leeuwenhoek. 1998;74(1–3):59–70. doi: 10.1023/a:1001799711799. [DOI] [PubMed] [Google Scholar]
- 24.Beal R, Betts WB. Role of rhamnolipid biosurfactants in the uptake and mineralization of hexadecane in Pseudomonas aeruginosa. Journal of Applied Microbiology. 2000;89(1):158–168. doi: 10.1046/j.1365-2672.2000.01104.x. [DOI] [PubMed] [Google Scholar]
- 25.Fonseca RR, Silva AJR, De França FP, Cardoso VL, Sérvulo EFC. Optimizing carbon/nitrogen ratio for biosurfactant production by a Bacillus subtilis strain. Applied Biochemistry and Biotechnology. 2007;137–140(1–12):471–486. doi: 10.1007/s12010-007-9073-z. [DOI] [PubMed] [Google Scholar]