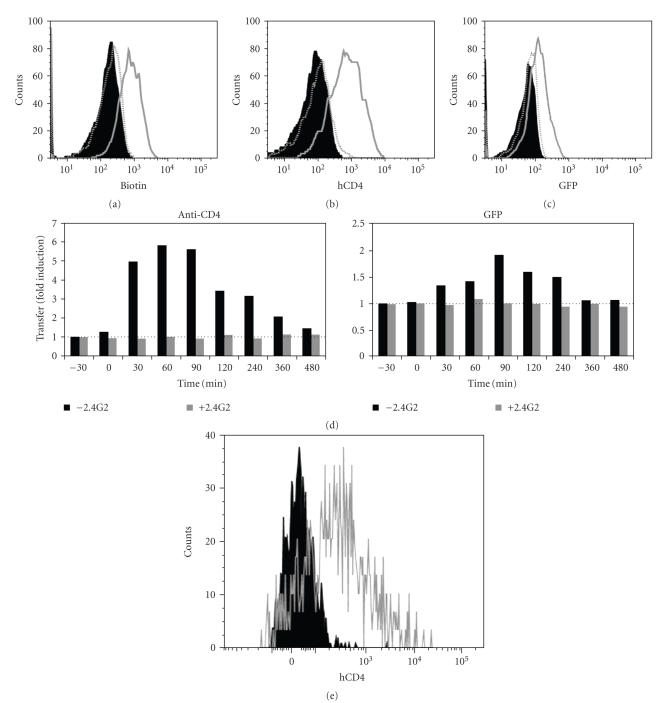
Figure 2.
CD8+ T cells from healthy donors and HIV+ patients capture CD4 and membrane fragments from P815-CD4-GFP cells during redirected trogocytosis. CD8+ T cells, immunomagnetically purified from human PBMC, were coated (grey open histogram) or not (black close histogram) with anti-CD3 + anti-CD28 mAb, then incubated with P815 cells expressing human CD4 fused to GFP, which had been previously cell surface biotinylated. (a) Cells were analyzed by flow cytometry for biotin capture using fluorescent streptavidin and CD8 expression. Shown are overlapping histograms of biotin detection on gated CD8+ T cells from healthy donor. Grey dotted open histogram shows biotin capture by anti-CD3 + anti-CD28-coated CD8+ T cells exposed to FcγRII/III-coated P815 cells previously coated by 2.4G2 mAb. (b) As in (a) except that CD4 rather than biotin capture was determined and is shown on gated CD8+ T cells from healthy donor. (c) As in (b) except that GFP rather than CD4 capture was determined and is shown on gated CD8+ T cells from healthy donor. (d) Kinetics of the transfer efficiency of CD4-GFP on CD8+ T cells co-incubated with P815-CD4-GFP. Transfer of CD4-GFP on CD8+ T cells was detected using anti-CD4 mAb (left panel) and GFP fluorescence (right panel). Results show the fold-induction, that is, the CD4 or GFP fluorescence on CD8+ T cells obtained from cocultures in the presence of stimulatory anti-CD3 + CD28 mAb divided by those obtained in the absence of stimulatory mAb. The experiments were performed in the presence (grey histograms) or absence (black histograms) of the blocking 2.4G2 mAb. (e) As in (b) except that CD8+ T cells were purified from PBMC of a-HIV-infected patient. Since we had only a few cells, we could not perform the control experiment with the blocking 2.4G2 mAb. Note that, in each case, similar results were obtained in at least three independent experiments.