Figure 1. Impaired UPRmt in haf-1 mutant animals.
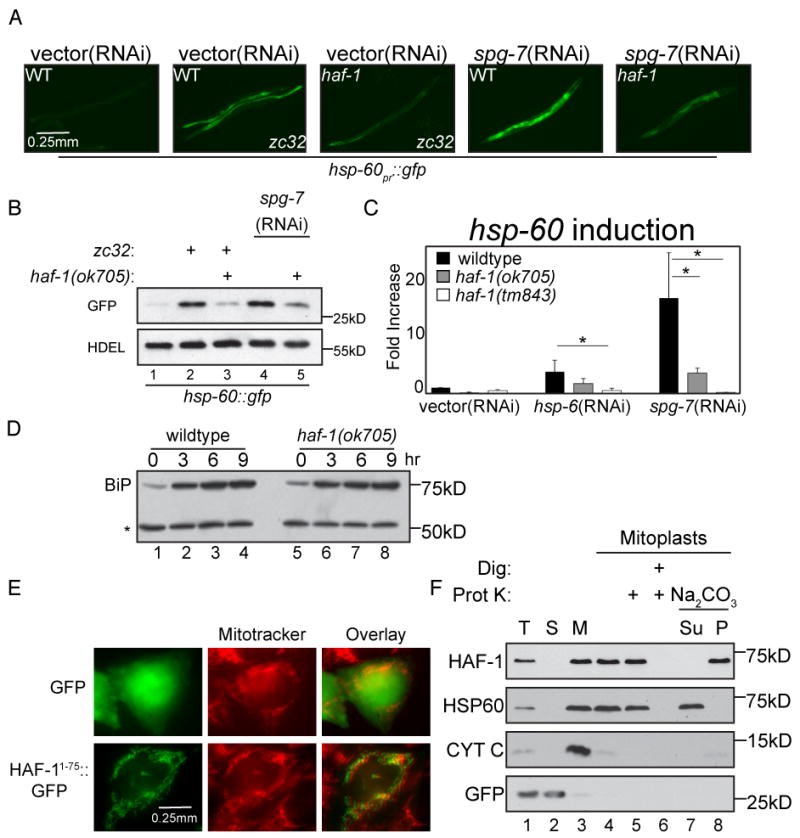
(A) Representative fluorescent photomicrographs of hsp-60pr∷gfp transgenic worms (reporting on the UPRmt) with a temperature sensitive mutation (zc32) that activates the UPRmt in an otherwise wildtype or haf-1(ok705) deleted background. Where indicated wildtype or haf-1(ok705) worms were raised on spg-7(RNAi) for 3 days to induce mitochondrial misfolded protein stress.
(B) A corresponding immunoblot of GFP expressed by hsp-60pr∷gfp transgenic worms in whom mitochondrial unfolded protein stress was induced (as in “A”). The endogenous ∼55kDa ER protein, detected with an anti-HDEL monoclonal antibody (lower panel) serves as a loading control.
(C) Quantitative analysis (by QRT-PCR) of endogenous hsp-60 mRNA in wildtype or two different haf-1 deleted strains (ok705 and tm843) subjected to mitochondrial unfolded protein stress by hsp-6(RNAi) or spg-7(RNAi). Displayed is the mean +/- SEM, n=3, *p<0.05.
(D) Immunoblot of proteins reactive with an anti-HDEL monoclonal antibody in extracts of wildtype and haf-1 mutant worms in which ER stress was induced by exposure to elevated temperature (30°C) for the indicated time. The upper band, BiP (HSP-4), is a UPRER target gene whereas the lower invariant ∼55Kd band (*) serves as a loading control.
(E) Fluorescent photomicrographs of Chinese Hamster Ovary (CHO) cells expressing GFP (upper panels) or GFP fused to amino acids 1-75 of HAF-1 (HAF-11-75∷GFP, lower panels). The cells were co-stained with the vital dye Mitotracker, which stains mitochondria (middle panels).
(F) Immunoblot of extracts from HEK293T cells expressing GFP (as a cytosolic marker) and C-terminally-tagged HAF-1∷FLAG following cellular fractionation into total lysate (T), post-mitochondrial supernatant (S) and mitochondrial pellet (M). Lanes 4-8 are from mitochondria treated with hypotonic buffer to generate mitoplasts and further treated with digitonin and proteinase K where indicated. Lanes 7-8 are mitoplasts incubated in Na2CO3 followed by centrifugation at 150,000 * g and separated into the pellet (P) and supernatant (Su).
