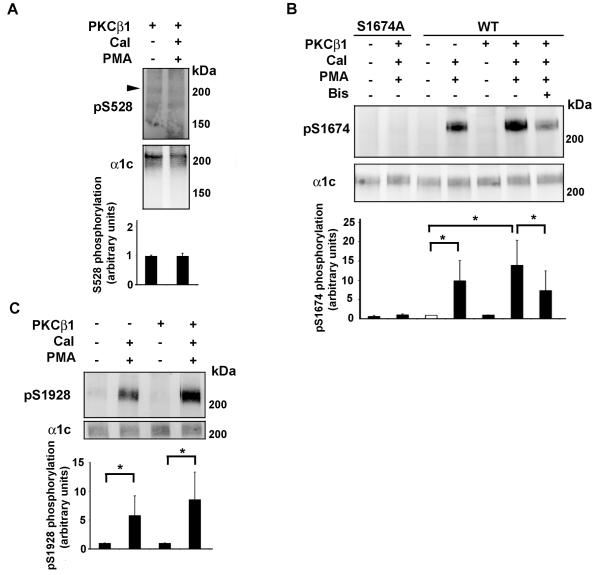
Figure 5. Reconstitution of PMA/PKC mediated phosphorylation of Ser1674 and Ser1928 in HEK cells.
A, Recombinant WT α1c was transiently co-expressed with β2a and PKCβ1 in HEK293 cells. Cells were exposed to PMA (1 μM) and calyculin A (Cal: 50 nM) for 10 min. Bisindolylmaleimide (Bis, 0.5 μM) was pre-incubated for 1 hr at 37°C. Cells were harvested 24-48 hours after transfection, and lysed in the presence of phosphatase inhibitors. Lysates were size-fractionated on SDS-polyacrylamide gel, transferred to nitrocellulose membranes, and blotted with anti-phospho-Ser528 (pS1928; upper panel) or α1c (middle panel) antibodies. Arrowhead indicates size of anticipated phosphorylated band. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA had no effect on Ser528 phosphorylation. Mean ± SD. B, Recombinant WT or Ala-substituted Ser1674 (S1674A) α1c was transiently co-expressed with β2a in HEK293 cells, in the absence or presence of PKCβ1 as indicated. Methodology is identical to (A) except nitrocellulose membranes were blotted with anti-phospho-Ser1674 (pS1674; upper panel) or α1c (middle panel) antibodies. The specificity of the pS1674 antibody is shown using the S1674A α1c mutant. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA increased phosphorylation of α1c Ser1674. Phosphorylation of Ser1674 was caused by PKC because bisindolylmaleimide (Bis) prevented Ser1674 phosphorylation. C, Recombinant WT or Ala-substituted Ser1928 (S1928A) α1c was transiently co-expressed with β2a in HEK293 cells, in the absence or presence of PKCβ1 as indicated. Methodology is identical to (A) except nitrocellulose membranes were blotted with anti-phospho-Ser1928 (pS1674; upper panel) or α1c (middle panel) antibodies. Representative of 3 similar experiments. Lower panel, Activation of PKC by PMA increased phosphorylation of α1c Ser1928. *, p<0.05