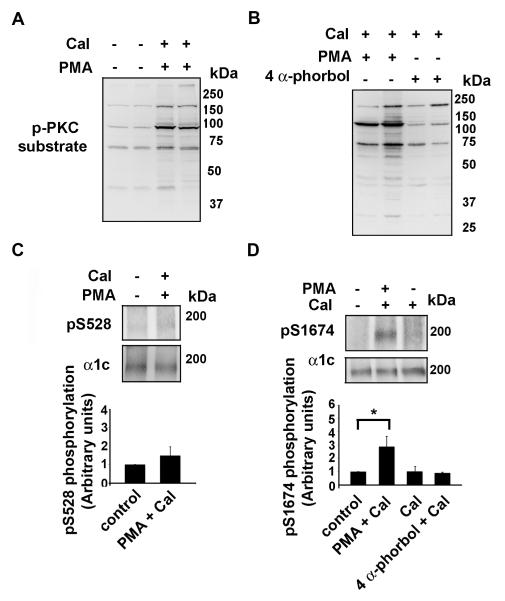
Figure 6. Ser1674 is phosphorylated in cardiomyocytes.
Rat hearts were perfused on a Langendorff apparatus with tyrode solution in the absence or presence of PMA (0.5 μM) + calyculin A (Cal, 50 nM) or 4 α-phorbol (0.5 μM) + calyculin A as indicated. Hearts were flash-frozen in liquid nitrogen and lysates prepared. A-B, Lysates were size-fractionated on SDS-PAGE, transferred to nitroceullose and blotted with anti-phospho-PKC substrate antibody. C-D, α1c immunoprecipates were size-fractionated on SDS-PAGE, transferred to nitrocellulose and blotted with anti-pS528 (upper panel) and anti-pS1674 antibodies (upper panel) and anti-α1c antibody (middle panels). Lower panels, bar graphs of densitometric quantification of Ser528 and Ser1674 phosphorylation (normalized to control; n=3-5). *, p < 0.025 PMA + calyculin compared to control; p<0.05 PMA + calyculin compared to calyculin A.