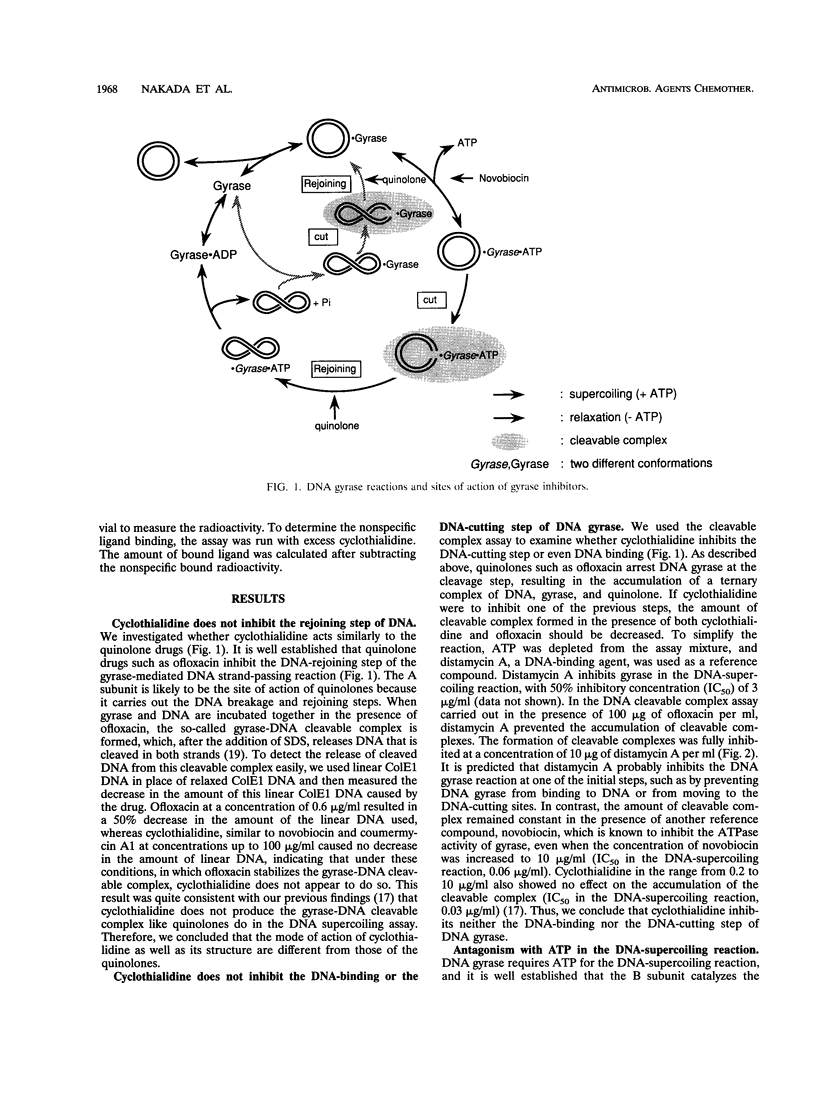
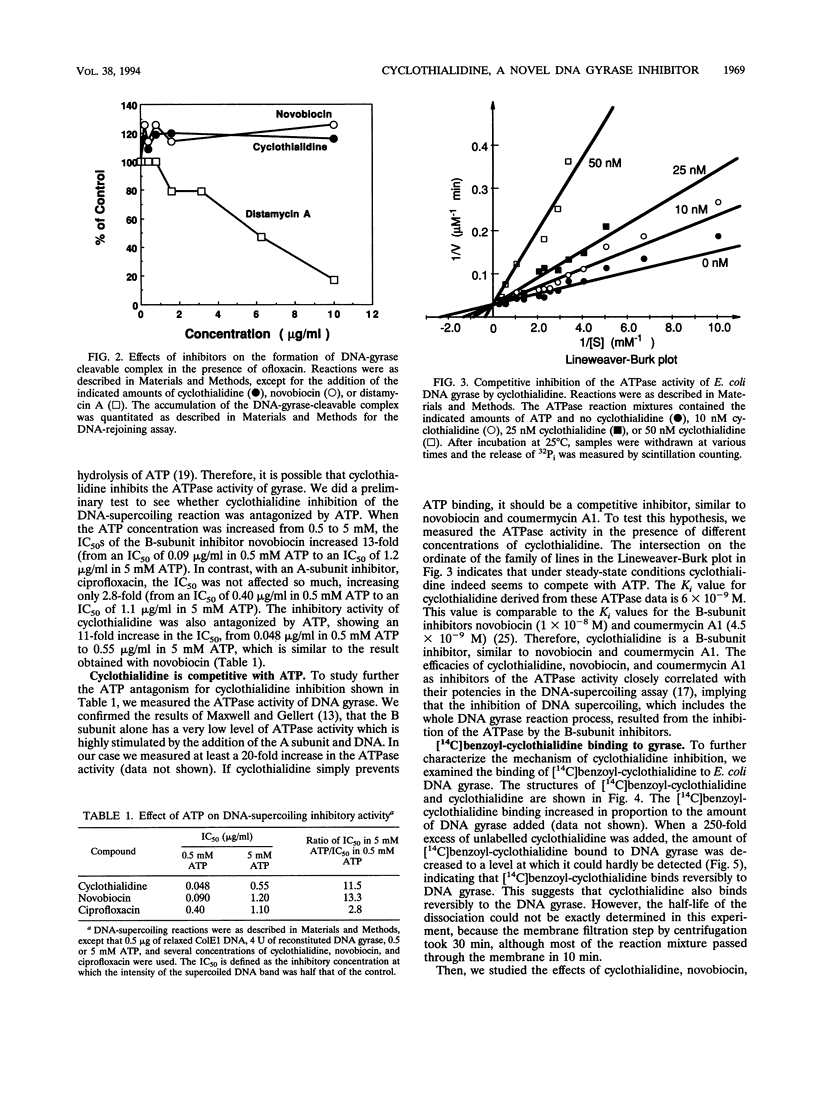
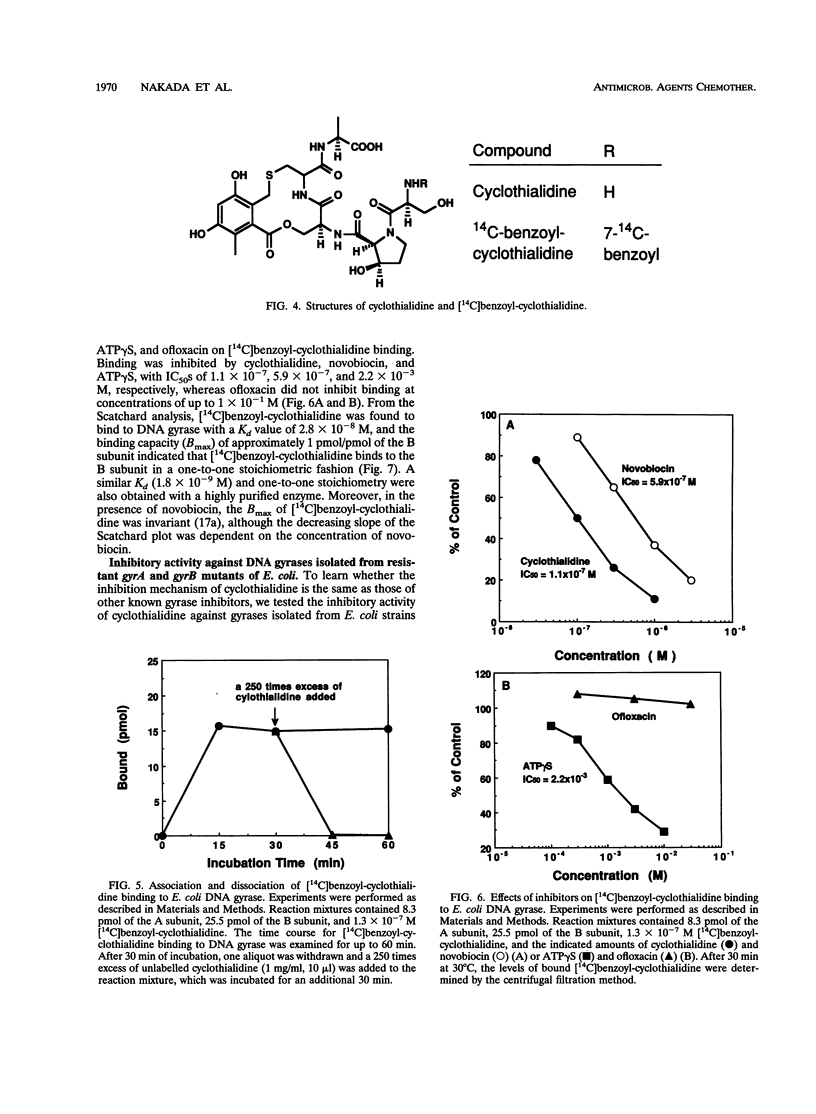
Abstract
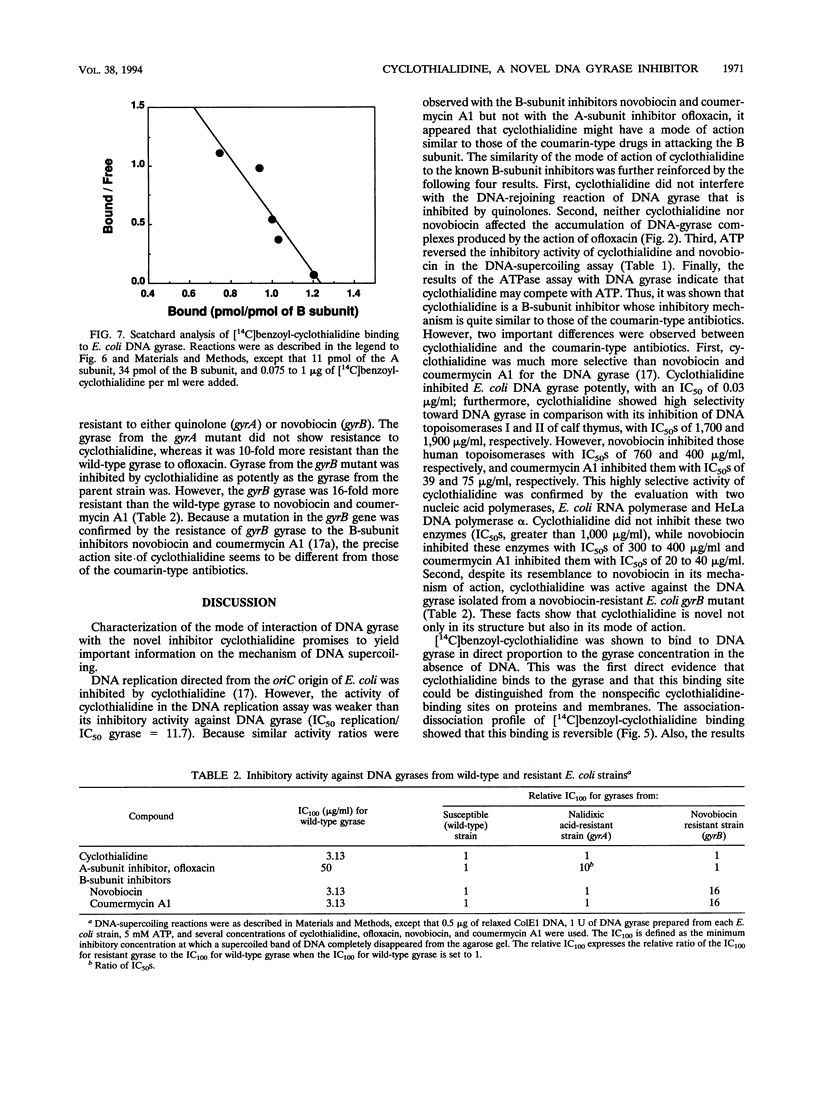
We investigated how cyclothialidine (Ro 09-1437), a novel DNA gyrase inhibitor belonging to a new chemical class of compounds, acts to inhibit Escherichia coli DNA gyrase. Cyclothialidine up to 100 micrograms/ml showed no effect on DNA gyrase when linear DNA was used as a substrate. Under the same conditions, quinolones, which inhibit the resealing reaction of DNA gyrase, caused a decrease in the amount of linear DNA used. No effect of cyclothialidine was observed on the accumulation of the covalent complex of DNA and the A subunit of DNA gyrase induced by ofloxacin in the absence of ATP. The effect of cyclothialidine on the DNA supercoiling reaction was antagonized by ATP, reducing the inhibitory activity 11-fold as the ATP concentration was increased from 0.5 to 5 mM. Cyclothialidine competitively inhibited the ATPase activity of DNA gyrase (Ki = 6 nM). The binding of [14C]benzoyl-cyclothialidine to E. coli gyrase was inhibited by ATP and novobiocin, but not by ofloxacin. These results suggest that cyclothialidine acts by interfering with the ATPase activity of the B subunit of DNA gyrase. Cyclothialidine was active against a DNA gyrase resistant to novobiocin, suggesting that its precise site of action might be different from that of novobiocin.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali J. A., Jackson A. P., Howells A. J., Maxwell A. The 43-kilodalton N-terminal fragment of the DNA gyrase B protein hydrolyzes ATP and binds coumarin drugs. Biochemistry. 1993 Mar 16;32(10):2717–2724. doi: 10.1021/bi00061a033. [DOI] [PubMed] [Google Scholar]
- Blobel G., Potter V. R. Nuclei from rat liver: isolation method that combines purity with high yield. Science. 1966 Dec 30;154(3757):1662–1665. doi: 10.1126/science.154.3757.1662. [DOI] [PubMed] [Google Scholar]
- Contreras A., Maxwell A. gyrB mutations which confer coumarin resistance also affect DNA supercoiling and ATP hydrolysis by Escherichia coli DNA gyrase. Mol Microbiol. 1992 Jun;6(12):1617–1624. doi: 10.1111/j.1365-2958.1992.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Drlica K., Franco R. J. Inhibitors of DNA topoisomerases. Biochemistry. 1988 Apr 5;27(7):2253–2259. doi: 10.1021/bi00407a001. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Mizuuchi K., O'Dea M. H., Itoh T., Tomizawa J. I. Nalidixic acid resistance: a second genetic character involved in DNA gyrase activity. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4772–4776. doi: 10.1073/pnas.74.11.4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M., O'Dea M. H., Itoh T., Tomizawa J. Novobiocin and coumermycin inhibit DNA supercoiling catalyzed by DNA gyrase. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4474–4478. doi: 10.1073/pnas.73.12.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschi E., Angehrn P., Gmuender H., Hebeisen P., Link H., Masciadri R., Nielsen J. Cyclothialidine and its congeners: a new class of DNA gyrase inhibitors. Pharmacol Ther. 1993 Nov;60(2):367–380. doi: 10.1016/0163-7258(93)90017-8. [DOI] [PubMed] [Google Scholar]
- Kamiyama T., Shimma N., Ohtsuka T., Nakayama N., Itezono Y., Nakada N., Watanabe J., Yokose K. Cyclothialidine, a novel DNA gyrase inhibitor. II. Isolation, characterization and structure elucidation. J Antibiot (Tokyo) 1994 Jan;47(1):37–45. doi: 10.7164/antibiotics.47.37. [DOI] [PubMed] [Google Scholar]
- Maxwell A., Gellert M. Mechanistic aspects of DNA topoisomerases. Adv Protein Chem. 1986;38:69–107. doi: 10.1016/s0065-3233(08)60526-4. [DOI] [PubMed] [Google Scholar]
- Maxwell A., Gellert M. The DNA dependence of the ATPase activity of DNA gyrase. J Biol Chem. 1984 Dec 10;259(23):14472–14480. [PubMed] [Google Scholar]
- Maxwell A. The interaction between coumarin drugs and DNA gyrase. Mol Microbiol. 1993 Aug;9(4):681–686. doi: 10.1111/j.1365-2958.1993.tb01728.x. [DOI] [PubMed] [Google Scholar]
- McCullough J. E., Muller M. T., Howells A. J., Maxwell A., O'Sullivan J., Summerill R. S., Parker W. L., Wells J. S., Bonner D. P., Fernandes P. B. Clerocidin, a terpenoid antibiotic, inhibits bacterial DNA gyrase. J Antibiot (Tokyo) 1993 Mar;46(3):526–530. doi: 10.7164/antibiotics.46.526. [DOI] [PubMed] [Google Scholar]
- Mizuuchi K., Mizuuchi M., O'Dea M. H., Gellert M. Cloning and simplified purification of Escherichia coli DNA gyrase A and B proteins. J Biol Chem. 1984 Jul 25;259(14):9199–9201. [PubMed] [Google Scholar]
- Nakada N., Shimada H., Hirata T., Aoki Y., Kamiyama T., Watanabe J., Arisawa M. Biological characterization of cyclothialidine, a new DNA gyrase inhibitor. Antimicrob Agents Chemother. 1993 Dec;37(12):2656–2661. doi: 10.1128/aac.37.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburne M. S., Maiese W. M., Greenstein M. In vitro inhibition of bacterial DNA gyrase by cinodine, a glycocinnamoylspermidine antibiotic. Antimicrob Agents Chemother. 1990 Jul;34(7):1450–1452. doi: 10.1128/aac.34.7.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otter R., Cozzarelli N. R. Escherichia coli DNA gyrase. Methods Enzymol. 1983;100:171–180. doi: 10.1016/0076-6879(83)00053-1. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Baranowski J., Pernet A. G. Mechanism of inhibition of DNA gyrase by quinolone antibacterials: specificity and cooperativity of drug binding to DNA. Biochemistry. 1989 May 2;28(9):3879–3885. doi: 10.1021/bi00435a038. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Kohlbrenner W. E., Weigl D., Baranowski J. Mechanism of quinolone inhibition of DNA gyrase. Appearance of unique norfloxacin binding sites in enzyme-DNA complexes. J Biol Chem. 1989 Feb 15;264(5):2973–2978. [PubMed] [Google Scholar]
- Shen L. L., Mitscher L. A., Sharma P. N., O'Donnell T. J., Chu D. W., Cooper C. S., Rosen T., Pernet A. G. Mechanism of inhibition of DNA gyrase by quinolone antibacterials: a cooperative drug--DNA binding model. Biochemistry. 1989 May 2;28(9):3886–3894. doi: 10.1021/bi00435a039. [DOI] [PubMed] [Google Scholar]
- Shen L. L., Pernet A. G. Mechanism of inhibition of DNA gyrase by analogues of nalidixic acid: the target of the drugs is DNA. Proc Natl Acad Sci U S A. 1985 Jan;82(2):307–311. doi: 10.1073/pnas.82.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staudenbauer W. L., Orr E. DNA gyrase: affinity chromatography on novobiocin-Sepharose and catalytic properties. Nucleic Acids Res. 1981 Aug 11;9(15):3589–3603. doi: 10.1093/nar/9.15.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Higgins N. P., Brown P. O., Peebles C. L., Cozzarelli N. R. Energy coupling in DNA gyrase and the mechanism of action of novobiocin. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4838–4842. doi: 10.1073/pnas.75.10.4838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugino A., Peebles C. L., Kreuzer K. N., Cozzarelli N. R. Mechanism of action of nalidixic acid: purification of Escherichia coli nalA gene product and its relationship to DNA gyrase and a novel nicking-closing enzyme. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4767–4771. doi: 10.1073/pnas.74.11.4767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutcliffe J. A., Gootz T. D., Barrett J. F. Biochemical characteristics and physiological significance of major DNA topoisomerases. Antimicrob Agents Chemother. 1989 Dec;33(12):2027–2033. doi: 10.1128/aac.33.12.2027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizán J. L., Hernández-Chico C., del Castillo I., Moreno F. The peptide antibiotic microcin B17 induces double-strand cleavage of DNA mediated by E. coli DNA gyrase. EMBO J. 1991 Feb;10(2):467–476. doi: 10.1002/j.1460-2075.1991.tb07969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J., Nakada N., Sawairi S., Shimada H., Ohshima S., Kamiyama T., Arisawa M. Cyclothialidine, a novel DNA gyrase inhibitor. I. Screening, taxonomy, fermentation and biological activity. J Antibiot (Tokyo) 1994 Jan;47(1):32–36. doi: 10.7164/antibiotics.47.32. [DOI] [PubMed] [Google Scholar]
- Wigley D. B., Davies G. J., Dodson E. J., Maxwell A., Dodson G. Crystal structure of an N-terminal fragment of the DNA gyrase B protein. Nature. 1991 Jun 20;351(6328):624–629. doi: 10.1038/351624a0. [DOI] [PubMed] [Google Scholar]
- del Castillo I., Vizán J. L., Rodríguez-Sáinz M. C., Moreno F. An unusual mechanism for resistance to the antibiotic coumermycin A1. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8860–8864. doi: 10.1073/pnas.88.19.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]