FIGURE 3. Purity of the OprF protein.
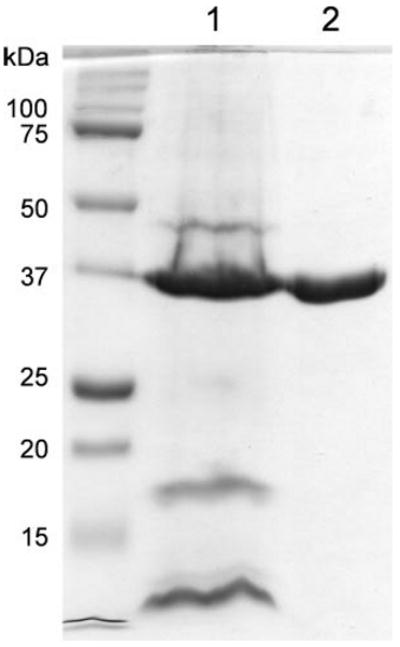
The starting material of the OprF purification (the crude outer membrane fraction) (lane 1) and the final OprF protein after fractionation on ion-exchange column and gel filtration (see “Experimental Procedures”) (lane 2) were separated by SDS-PAGE and stained with Coomassie Blue. Lane 1 contained molecular weight standards. The doublet pattern of OprF (lane 2) is characteristic of this protein and is caused partly by the difficulty in denaturing the N-terminal β-barrel structure (20) and perhaps also by the partial reduction of the two disulfide bonds present in OprF (see the legend for Fig. 5). We applied 5 μg of OprF preparation to show the absence of contaminating proteins. Densitometric scanning of gels to which less OprF was applied showed that this preparation of OprF was at least 97% pure.
