Table 2.
Catalytic enantioselective crotylation of N-benzyl isatins 1a–1g via iridium catalyzed C-C bond forming transfer hydrogenation.
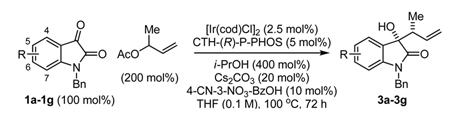 | ||||
|---|---|---|---|---|
| Entry | Isatins 1a–1g | Products | Yield (%) | ee (%), dr |
| 1b | 1a, N-benzyl isatin | 3a | 83 | 80, 13:1 |
| 2f | 1b, 5-bromo-N-benzyl isatin | 3b | 72 | 86, 16:1 |
| 3d,e | 1c, 5-methyl-N-benzyl isatin | 3c | 81 | 89, 18:1 |
| 4d,e | 1d, 5-methoxy-N-benzyl isatin | 3d | 87 | 92, 29:1 |
| 5b | 1e, 6-chloro-N-benzyl isatin | 3e | 70 | 91, 19:1 |
| 6f | 1f, 6-bromo-N-benzyl isatin | 3f | 81 | 89, 15:1 |
| 7b,c | 1g, 7-fluoro-N-benzyl isatin | 3g | 64 | 85, 19:1 |
As described in Table 2 footnotes.
10 mol% loading of Cs2CO3 was used.
400 mol% loading of allyl acetate was used.
Me-THF was used as solvent.
The reaction was run for 40 hours.
5 mol% loading of [Ir(cod)Cl]2, 10 mol% loading of CTH-(R)-P-PHOS and 20 mol% loading of 4-CN-3-NO2-BzOH were used.
