Table 3.
Conformation of Pro and its 4-substituted derivatives that prefer the Xaa position [φ = −75°, ψ = 164° (7)] in a collagen triple helix.
| Residue (References) | Crystal structure |
Ring pucker |
(Eendo− Eexo)a (kcal/mol) |
φ (degrees) |
ψ degrees) |
Ktrans/cisc | |
|---|---|---|---|---|---|---|---|
| Pro (54, 56, 57) | 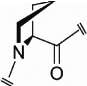 |
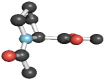 |
Cγ- endo |
−0.41 | −79b | 177b | 4.6 |
| mcp (66) | 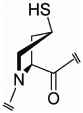 |
–- | Cγ- endo |
–- | –- | –- | 4.7 |
| mep (65) | 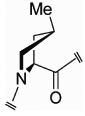 |
–- | Cγ- endo |
−1.4 | –- | –- | 3.7 |
| flp (54, 56, 68) |  |
–- | Cγ- endo |
−0.61 | −76a | 172a | 2.5 |
| clp (61) |  |
–- | Cγ- endo |
–- | –- | –- | 2.2 |
From DFT calculations.
Values of φ and ψ (here, Ni–Cα i–C′i–Oi+1) are from the crystal structure of Ac-Pro-OMe, which has a cis peptide bond.
Values of Ktrans/cis(Figure 5) were determined in solution by NMR spectroscopy.
