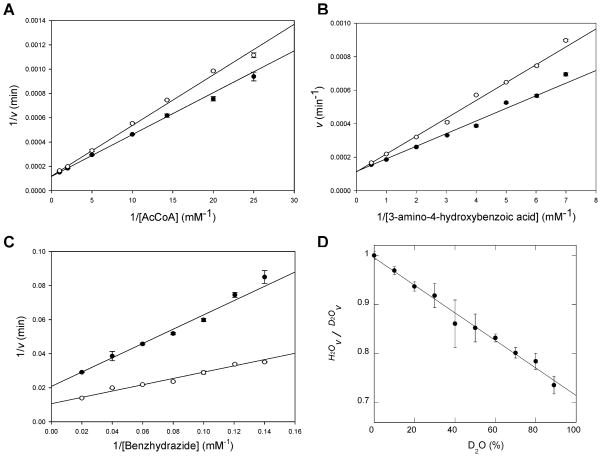
Figure 3.
Solvent kinetic isotope effects. (A) AHB was used at a fixed concentration of 2.5 mM, and the concentration of AcCoA was varied. Points are experimental, while the lines are fits of the data to eq 6 yielding the following values: D2Okcat = 1.0 ± 0.1 and D2O(kcat/KAcCoA) = 0.64 ± 0.03. (B) AcCoA was used at a fixed concentration of 1.5 mM, and the concentration of AHB was varied. Points are experimental, while the lines are fits of the data to eq 6 yielding the following values: D2Okcat = 1.0 ± 0.1 and D2O(kcat/KAHB) = 0.72 ± 0.06. (C) AcCoA was used at a fixed concentration of 1.5 mM, and the concentration of benzoic acid hydrazide was varied. Points are experimental, while the lines are fits of the data to eq 6 yielding the following values: D2Okcat = 1.9 ± 0.2 and D2O(kcat/KBAH) = 2.3 ± 0.3. For panels A–C, all data represent averages of two independent experiments. White circles represent data obtained in H2O, and black circles represent data measured in 98% D2O. (D) Proton inventory. Amine substrate (AHB) and AcCoA concentrations were fixed at 0.16 and 0.072 mM, respectively. The percentage of D2O was varied from 0 to 88%; each assay was performed in triplicate, and standard deviations are shown as error bars.