Abstract
Background
Previous studies have demonstrated a specific cognitive bias for sad stimuli in currently depressed patients; little is known, however, about whether this bias persists after recovery from the depressive episode. Depression is frequently observed in patients with asthma and is associated with a worse course of the disease. Given these high rates of co-morbidity, we could expect to observe a similar bias towards sad stimuli in patients with asthma.
Method
We therefore examined cognitive biases in memory and attention in 20 currently and 20 formerly depressed participants, 20 never-depressed patients diagnosed with asthma, and 20 healthy control participants. All participants completed three cognitive tasks: the self-referential encoding and incidental recall task, the emotion face dot-probe task and the emotional Stroop task.
Results
Compared with healthy participants, currently and formerly depressed participants, but not patients with asthma, exhibited specific biases for sad stimuli.
Conclusions
These results suggest that cognitive biases are evident in depression even after recovery from an acute episode but are not found in never-depressed patients with asthma.
Keywords: Asthma, depression, emotion, information processing, remission
Introduction
Cognitive models of depression, such as schema theories (Beck, 1967, 1976) and associative network models (e.g. Bower, 1981), emphasize the role of dysfunctional cognitive structures and cognitive biases in virtually all aspects of information processing, including perception, attention and memory, in the onset and maintenance of this disorder. In a metaanalysis, Beck & Perkins (2001) demonstrated that depressed patients attend selectively to, and have better memory for, schema-congruent than schema-incongruent information. Moreover, depressed individuals exhibit better recall for depression-specific than for neutral stimuli (Moritz et al. 2005) and recall more negative than positive stimuli (Matt et al. 1992; Gotlib et al. 2004b). In contrast, non-depressed individuals recall more positive than negative material (Matt et al. 1992). In the directed forgetting task depressed participants showed retrieval facilitation for to-be-forgotten negative words than for positive material, whereas this effect did not appear in clinically anxious patients and healthy controls (Power et al. 2000). In a go/no-go task depressed patients made more omission errors during happy than sad word blocks and required more time to respond to happy than to sad words, whereas healthy controls needed more time to respond to sad than to happy words (Erickson et al. 2005).
Using the emotional dot-probe task, attentional biases to depression-specific words have been found consistently in individuals with anxiety disorder (e.g. Gotlib & McCann, 1984; Mogg et al. 1992, 1995; Mathews et al. 1996; Gotlib et al. 2004a, b). In contrast, for currently depressed patients, a bias for negative words is generally found only if the stimuli are presented for 1000 ms or longer (e.g. Gotlib & Cane, 1987; Mogg et al. 1995; Bradley et al. 1997; Gotlib et al. 2004a, b). Other studies using dot-probe tasks indicate that depressed individuals do not exhibit the attentional bias for positive stimuli that was found in healthy participants (e.g. Gotlib et al. 1998). Given these findings, Bradley et al. (1997) suggested that depression might not be associated with an initial orienting bias towards negative stimuli, but rather, once that information has become the focus of attention depressed participants might have greater difficulties in disengaging their attention from it. Consistent with this hypothesis, studies demonstrated a content-specific bias to sad faces presented for 1000 ms in acutely depressed participants, but not in patients with generalized anxiety disorder (Bradley et al. 1997; Gotlib et al. 2004a, b). With neuropsychological tests of memory and planning ability Murphy et al. (1999) showed an affective bias for negative stimuli and impairment in the ability to shift the focus of attention in patients with depression. Furthermore, Mogg et al. (2000) observed no bias in clinically depressed participants who also met criteria for generalized anxiety disorder. Overall, only a few studies examining attentional biases to sad faces in clinically depressed patients have excluded patients with a diagnosis of co-morbid anxiety disorder (Gotlib et al. 2004a, b; Joormann & Gotlib, 2007).
The Stroop task (Stroop, 1935) assesses attentional interference; biases to threatening stimuli in this task are well documented for participants diagnosed with anxiety disorders (Mogg et al. 1993). Interference effects in depressed patients, however, are reported less consistently. Although attentional interference to depression-specific words has been demonstrated in some studies examining participants with current depression (e.g. Gotlib & McCann, 1984; Gotlib & Cane, 1987), other studies found no association between reaction time and depression in the emotional Stroop task (Gilboa & Gotlib, 1997).
Most of the findings described above were obtained in currently depressed patients, whereas little is known about cognitive biases following recovery from a depressive episode. Beck (1967, 1976) postulated that cognitive patterns are stable and, therefore, that cognitive biases should also be evident in formerly depressed patients. The fact that almost 80% of individuals diagnosed with depression experience more than one depressive episode (Boland & Keller, 2002) supports this assumption. Initial studies suggested that increased vulnerability for recurrent depressive episodes in formerly depressed individuals is associated with depression-specific schemas (Segal et al. 1999), dysfunctional patterns of thought (e.g. Gilboa & Gotlib, 1997; McCabe et al. 2000) and with depression-specific memory biases (Hedlund & Rude, 1995; Gotlib et al. 2000; Joormann & Gotlib, 2007). Other studies, however, have not found evidence for cognitive biases in formerly depressed patients (Blackburn et al. 1986; Gotlib & Cane, 1987).
In sum, for currently depressed individuals consistent support has been obtained for negative biases in memory; the evidence for attentional biases, however, has been mixed. Because nearly all of these studies used only one task to assess biases, it is difficult to determine whether inconsistent results are attributable to differences among tests, study designs or participant groups. Additionally, only few studies conducted thorough diagnosis of depressed participants to exclude co-morbid anxiety disorders (Gotlib et al. 2004a, b; Joormann & Gotlib, 2007), which appear to be associated with different patterns of cognitive biases. Finally, it is unclear whether these biases continue operating after remission from a depressive episode and, thus, constitute a risk factor for symptom recurrence.
Asthma is a common chronic respiratory disease that is associated with recurrent episodes of cough, bronchoconstriction and breathlessness, leading to reduced quality of life (Global Initiative for Asthma, 2007). Depression is a highly prominent co-morbid condition in asthma patients (Zielinski & Brown, 2003). Reported prevalence rates reach up to 41%, which is not only higher than in healthy participants, but also higher than in other conditions such as arthritis or heart disease (Dunlop et al. 2004). Goodwin et al. (2004) examined adolescents and young adults and demonstrated that the relationship between asthma and depressive symptoms may reflect effects of common factors like exposure to childhood adversity rather than a direct causal link. Depression in asthma is related to worse course of disease, including more hospitalizations, higher oral corticosteroid intake, elevated symptoms and functional disability as well as work absence (Allen et al. 1994; Stein et al. 2006; Kullowatz et al. 2007). Several studies have demonstrated that negative emotions are associated with decreased lung function in asthma patients (Ritz et al. 2000; Ritz & Steptoe, 2000; von Leupoldt & Dahme, 2005; von Leupoldt et al. 2006). The reasons for the high prevalence of depression in asthma are still unknown; unfortunately, few studies have gone beyond simply describing rates of co-morbidity to examine this association. It is possible, however, that patients with asthma exhibit cognitive biases for sad stimuli similar to those found in patients with depression, which may constitute a risk factor for the development of depressive symptoms.
In the present study we examined whether formerly depressed individuals and patients with asthma exhibit cognitive biases similar to those observed in currently depressed individuals. In addition, we compared these groups with healthy participants. Three different cognitive tests were used to study different aspects of information processing: selective perception, attention and recall.
Method
Participants
Four groups of participants were examined: 20 patients diagnosed with current major depressive disorder (MDD), 20 participants with at least one diagnosed depressive episode in their lifetime who were currently remitted (RMD), 20 participants with physician-diagnosed asthma without current or former depression and 20 healthy non-psychiatric controls (NC) (Table 1). To ensure the homogeneity of the group of MDD participants, they were recruited from a medical and psychosomatic hospital at the beginning of their in-patient stay. RMD and NC participants were recruited by local newspaper advertisements and flyers posted at the University of Hamburg. Asthma patients were recruited from an out-patient Pulmonary Research Institute. Participants were included in the MDD group if they met the Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV; Saß et al. 1996) criteria for a current major depressive episode. Participants were included in the RMD group if they met DSM-IV criteria for a past major depressive episode. To confirm full recovery from depression, participants in the RMD group underwent a structured interview based on the DSM-IV (Joormann & Gotlib, 2007). They were asked for the degree of depressive symptoms they experienced during the previous 8 weeks using guidelines recommended by the National Institute of Mental Health Collaborative Program of the Psychobiology of Depression (e.g. Keller et al. 1992): 8 consecutive weeks with no more than two symptoms of no more than a mild degree. MDD and RMD participants were excluded in case of severe head trauma, learning disabilities, current/past anxiety disorders, psychotic symptoms, bipolar disorder, alcohol or substance abuse within the previous 12 months, and asthma symptoms. Current antidepressive medication in MDD and RMD participants was no exclusion criteria (MDD, n = 2; RMD, n = 6). Similar exclusion criteria were used for the asthma and healthy control groups. Participants with physician-diagnosed asthma were included when presenting mild to moderately severe asthma according to guidelines of the Global Initiative for Asthma (2007). Asthma patients were excluded if meeting criteria for current or former depression. The four groups were matched for age, gender and level of education. All participants gave informed consent and the local ethics committee approved the study protocol.
Table 1.
Demographic and clinical characteristics of the sample
| Group |
||||
|---|---|---|---|---|
| Asthma | MDD | RMD | NC | |
| Gender, n | ||||
| Male | 10 | 10 | 10 | 10 |
| Female | 10 | 10 | 10 | 10 |
| Age, years | 39.15 (8.43) | 40.60 (9.23) | 39.95 (11.62) | 38.45 (7.69) |
| Educationd | 3.35 (1.31) | 3.10 (1.55) | 2.95 (1.05) | 3.50 (0.95) |
| SAM valence | ||||
| SRET | 3.45 (1.23) | 2.65 (1.31) | 2.85 (1.27) | 3.30 (2.00) |
| Dot-probe | 3.60 (1.31) | 3.00 (1.52) | 3.05 (1.47) | 3.45 (1.64) |
| Stroop | 3.90 (1.41) | 2.65 (1.50) | 3.00 (1.34) | 3.90 (1.89) |
| SAM arousal | ||||
| SRET | 4.00 (2.33) | 5.90 (2.00) | 5.10 (2.17) | 3.95 (2.13) |
| Dot-probe task | 4.10 (2.34) | 5.15 (2.01) | 5.05 (2.16) | 4.35 (2.08) |
| Stroop task | 4.00 (2.29) | 5.25 (2.43) | 4.75 (1.10) | 4.00 (1.95) |
| ADS | 6.95 (4.89)a,c | 28.40 (13.72)b | 10.65 (5.16)c | 4.85 (5.11)a |
| BDI | 3.35 (3.36)a | 21.05 (11.36)b | 8.75 (6.72)c | 2.50 (5.34)a |
| BAI | 4.55 (5.10)a | 10.25 (5.60)b | 5.75 (5.23)c | 1.90 (2.94)a |
| BSI-GSI, T score | 47.60 (10.71)a | 72.05 (7.97)b | 58.85 (12.58)c | 41.45 (9.67)a |
MDD, Major depressive disorder; RMD, remitted depressed; NC, non-psychiatric healthy controls; SAM, Self-Assessment Manikin (dimensions valence and arousal); SRET, Self-Referential Encoding and Incidental Recall Task; ADS, Allgemeine Depressionsskala; BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; BSI, Brief Symptom Inventory; GSI, Global Severity Index.
Values are given as mean (standard deviation) unless otherwise indicated.
Different superscripts within rows indicate significant group differences (p ≤ 0.05).
Education was assessed on a four-point scale, with higher numbers representing a higher level of education.
Diagnostic assessment
The Structured Clinical Interview for DSM-IV (SCID, German adaptation; Wittchen et al. 1997) was used to confirm presence or absence of current or former depressive episodes and any other Axis I disorders. A trained psychologist conducted the interview. Prior to SCID administration, participants completed the German versions of the Beck Depression Inventory (BDI, German adaptation; Hautzinger et al. 1994), the Beck Anxiety Inventory (BAI; Margraf & Ehlers, 2007), the Center for Epidemiological Studies Depression Scale (Allgemeine Depressionsskala, ADS; Hautzinger & Bailer, 1993) and the Brief Symptom Inventory (BSI; Franke, 2000) to assess the severity of acute symptoms of depression, anxiety and other psychological symptoms.
Stimuli for information processing tasks
Self-referential encoding and incidental recall task (SRET)
A set of 20 depressotypic, 20 socially threatening, 20 physically threatening and 50 positive adjectives was derived from previous studies of information processing in depression (Gotlib et al. 2004a, b). Additional adjectives were selected from the Handbook of German Affective Word Norms (Hager & Hasselhorn, 1994). All words were matched for valence and arousal. Five psychologists and psychotherapists rated the German stimulus words with regard to relevance for depression, social and physical threat and positive emotions. With at least a 4:1 agreement, words were included in the appropriate category.
Emotion face dot-probe task
Similar to previous studies (Gotlib et al. 2004a; Joormann & Gotlib, 2007), a set of 20 photographs of faces of people posing sad, happy and neutral expression was used from the MacArthur Face Stimuli Set (http://www.macbrain.org/faces/index.htm). From this validated set of 646 photos with faces exhibiting different facial expressions (Tottenham et al. 2002), an equal number of male and female faces that each posed a neutral, happy and sad expression were selected for the current study, as well as an equal number of faces of different ethnicities.
Emotional Stroop task
Three sets of 24 words matched for length, frequency and word class were used: depression-specific, positive and neutral words (eight words per category). The words were chosen from previous studies examining cognition and emotion (e.g. Gotlib & McCann, 1984; Bradley & Mathews, 1988; Gotlib et al. 2004a, b). The German version of the word lists was validated and successfully applied in a previous study examining attentional and memory biases in depression and social phobia (Rinck & Becker, 2005).
Procedure
In the first session participants completed clinical interviews and questionnaires. In a second session 2 h later, they completed the information-processing tasks presented on a notebook [screen 15.4 inches (39.1 cm)] in the same fixed order (SRET, dot-probe task, Stroop test) to ensure that verbal and non-verbal tasks were alternated and that no retroactive interference would occur on the incidental recall task. Each test consisted of a practice and a test trial. Micro Experimental Laboratory (MEL) software (e-prime v. 1.1; Psychology Software Tools, Inc., USA) and a response box with a MEL voice-activated microphone were used for stimulus presentation and recording of response accuracy/latency. Recent studies demonstrated that an initial mood induction is necessary to detect cognitive biases in formerly depressed participants (Gilboa & Gotlib, 1997; McCabe et al. 2000). Before each task we, therefore, presented one of three picture sets, each including 12 pictures of sad scenes (each picture presented for 10 s). Pictures were selected from the International Affective Picture System (Lang et al. 1999), which is a validated instrument for emotion induction (Bradley & Lang, 2000) and includes normative ratings for valence (pleasant–unpleasant) and arousal (high–low). After each picture series, participants rated their current mood on the affective dimensions of valence and arousal using the Self-Assessment Manikin (Lang et al. 1980). The participants completed the tasks after practice trials in the absence of the experimenter.
SRET
Each trial started with the phrase ‘Describes me?’ presented for 500 ms and after a pause of 250 ms one of the stimuli words was presented in randomized order. By pressing an appropriate key labelled with ‘yes’ or ‘no’ participants indicated whether the displayed word described them. Then the word disappeared and the next ‘Describes me?’ followed. With the second part of the SRET participants were asked to recall as many words as possible from the previous self-referential encoding task within 3 min, regardless of whether or not they endorsed the words as self-descriptive.
Emotion face dot-probe task
Each of the 20 happy and 20 sad faces were paired with a neutral face of the same actor. These 40 pairs of pictures were presented in randomized order four times (each time 1000 ms), for a total of 160 trials. Each trial started with a fixation cross (1000 ms). When the pictures disappeared, a dot was presented either on the side where the emotional face or the neutral face had been presented before. Participants had to indicate via pressing a key labelled with ‘right’ or ‘left’ the location of the dot as quickly and accurately as possible. With equal probability both the emotional face of the same actor and the dot appeared in the left or right position.
Emotional Stroop task
Following the presentation of a fixation cross (500 ms) and a subsequent pause (500 ms) the words of the three sets were presented in random order and assigned randomly to appear in red, green, blue and yellow. Participants were instructed to name only the colour of the word and to ignore its meaning. The latencies from stimulus presentation to the participants’ colour-naming responses, which activated the offset of the word, were recorded by the MEL voice-activated microphone and response box.
Measures
SRET
The bias score was calculated as the number of originally endorsed and subsequently recalled words from each content category, divided by the total number of words endorsed and recalled (Gotlib et al. 2004b). Reaction time is another index of cognitive biases (Gotlib et al. 2004a, b), and was calculated by the mean latency to make a decision for the words in each content category.
Emotion face dot-probe task
The dot-probe bias score was calculated by subtracting the mean probe detection times for probes appearing in the same position as the emotional face from the mean probe detection times for probes appearing in a different position than the emotional face. Positive values of this bias score indicate a shift of attention towards the spatial location of emotional faces relative to matched neutral faces, and negative values indicate a shift of attention away from the spatial location of emotional faces relative to matched neutral faces (Mogg et al. 1995).
Emotional Stroop task
Bias scores were calculated by subtracting the mean reaction time for words in the neutral words condition from the mean reaction time for words in each emotional condition. Higher scores indicated greater interference and, thus, greater cognitive bias (Gotlib et al. 2004a).
Analyses
Group means for bias scores in all three tasks were analysed with repeated-measures analyses of variance (ANOVAs). To achieve comparability with previous studies (Gotlib et al. 2004a, b), these ANOVAs were followed by Fisher’s least significance difference post-hoc tests. All analyses were calculated with SPSS 15.0 software (SPSS Inc., USA) using a significance level of p < 0.05.
Results
Demographic and clinical characteristics
Group characteristics are presented in Table 1. The four groups did not differ with respect to age [F(3, 79) = 0.20, p < 1], education [F(3, 79) = 0.68, p < 1] and female:male ratio. As expected, the four groups differed in clinical variables. One-way ANOVAs yielded effects for groups in the ADS [F(3, 79) = 34.74, p < 0.001], BDI [F(3, 79) = 27.10, p < 0.001], BAI [F(3, 79) = 10.38, p < 0.001] and BSI-Global Severity Index (GSI) [F(3, 79) = 27.79, p < 0.001]. Post-hoc tests indicated that the MDD group scored higher on each of these measures compared with the RMD, NC and asthma groups (BAI, p < 0.05, all others, p < 0.001). The RMD group exhibited higher scores than the NC in the ADS, BDI, BAI and BSI-GSI (all p < 0.05) and higher scores than the asthmatics in the BDI and BSI-GSI (both p < 0.05), but lower scores than the MDD group (all p < 0.001). However, neither participants of the asthma group nor of the RMD and the NC groups reached the clinically relevant cut-off scores for depression and anxiety (Table 1). Analyses of valence ratings after the affective picture series using a 4 (diagnostic group) × 3 (mood induction series) ANOVA yielded an effect for the three mood inductions across all groups [F(2, 152) = 3.13, p < 0.05]. Valence was lowest before the SRET task and highest before the Stroop task (Table 1). No effects were obtained for arousal ratings.
Group differences in cognitive tasks
SRET
To analyse the SRET bias we conducted two separate analyses, because the proportions necessarily sum to 1.0, which prevents inclusion of all four emotion categories in a single ANOVA. For the three categories of endorsed and subsequently recalled words the 4 (diagnostic group) × 3 (negative emotion category) repeated-measures ANOVA yielded an interaction of diagnostic group and negative emotion category [F(6, 152) = 5.40, p < 0.001], and an effect for emotional category [F(2, 152) = 15.02, p < 0.001] as well as for diagnostic group [F(3, 76) = 26.51, p < 0.001]. Post-hoc tests showed that both the MDD and RMD groups recalled more endorsed sad words than socially and physically threatening words (all p < 0.05), whereas the NC and asthma groups did not differ with respect to all three negative categories (Table 2). Post-hoc tests of the emotion main effect indicated that across all groups a higher proportion of sad than social (p < 0.001) and physically threatening endorsed words (p < 0.05) were recalled, with social and physically threatening words not differing from each other. Post-hoc tests of the group main effect revealed that the MDD and RMD groups recalled more endorsed negative words than the NC and asthma groups (all p < 0.001). The MDD group showed a stronger bias to negative adjectives than the RMD group (p < 0.05).
Table 2.
Bias scores on the SRET
| Group |
||||
|---|---|---|---|---|
| Category | Asthma | MDD | RMD | NC |
| Proportion of words endorsed | ||||
| sad | 0.04 (0.04)a | 0.27 (0.20)b | 0.13 (0.12)c | 0.03 (0.04)a |
| pos | 0.88 (0.10)a | 0.46 (0.19)b | 0.69 (0.17)c | 0.92 (0.10)a |
| pt | 0.03 (0.04)a | 0.12 (0.07)b | 0.08 (0.05)c | 0.02 (0.03)a |
| st | 0.05 (0.04)a | 0.16 (0.07)b | 0.09 (0.05)c | 0.03 (0.04)a |
| Mean reaction time to words, ms | ||||
| sad | 1400.9 (507.0) | 1987.2 (702.8)a | 1915.3 (591.1)a | 1472.1 (529.9) |
| pos | 1361.3 (494.0)a | 2664.1 (3081.6)b | 1707.1 (645.2)a,b | 1364.3 (489.4)a |
| pt | 1288.4 (424.1) | 1915.8 (526.1)a | 1778.2 (608.6)a | 1342.3 (497.6) |
| st | 1453.7 (461.4)a,c | 2063.6 (528.1)b | 1888.4 (627.0)b,c | 1575.1 (607.0)a |
| total | 1372.1 (446.7) | 1978.0 (515.8)a | 1790.8 (519.1)a | 1418.2 (490.1) |
| Proportion of endorsed and recalled words | ||||
| sad | 0.02 (0.05) | 0.37 (0.27)a | 0.31 (0.33)a | 0.02 (0.04) |
| pos | 0.91 (0.11) | 0.40 (0.27)a | 0.56 (0.36)b | 0.95 (0.08) |
| pt | 0.04 (0.08) | 0.13 (0.22)a | 0.05 (0.07)b | 0.02 (0.05) |
| st | 0.03 (0.06)a,c | 0.11 (0.11)b | 0.09 (0.14)b,c | 0.01 (0.03)a |
SRET, Self-Referential Encoding and Incidental Recall Task; MDD, major depressive disorder group; RMD, remitted depressed group; NC, non-psychiatric healthy control group; sad, depression-specific words; pos, positive words; pt, physically threatening words; st, socially threatening words.
Values are given as mean (standard deviation).
Different superscripts within rows indicate significant group differences (p ≤ 0.05).
The one-way ANOVA conducted on positive adjectives yielded an effect for diagnostic group [F(3, 76) = 26.42, p < 0.001]. Post-hoc tests revealed that both the MDD and RMD groups recalled a lower proportion of endorsed positive words than the NC and asthma groups (both p < 0.001), which did not differ from each other. The RMD group recalled more endorsed positive words than the MDD group (p < 0.05).
Reaction times for self-referential decisions were analysed with a 4 (diagnostic group) × 3 (emotion category) repeated-measures ANOVA, which yielded a main effect for diagnostic group [F(3, 76) = 7.17, p < 0.001]. Post-hoc tests demonstrated that both the MDD group (p < 0.001) and the RMD group (p < 0.05) required more time to decide than the NC and asthma groups, which did not differ from each other (Table 2).
Emotion face dot-probe task
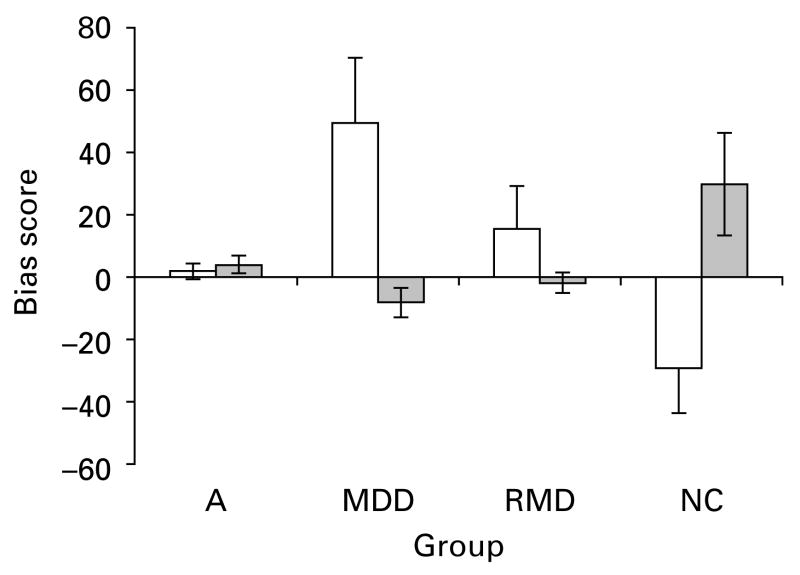
A 4 (diagnostic group) × 2 (emotion category) repeated-measures ANOVA yielded an interaction of diagnostic group and emotion category [F(3, 76) = 5.95, p = 0.001] (Fig. 1). As expected, post-hoc tests demonstrated that both the MDD (p < 0.001) and RMD groups (p < 0.05), which did not differ from each other, were faster in detecting the dot probes behind sad faces than the NC group (Table 3). Moreover, the MDD participants demonstrated a higher bias score for sad faces than the asthma group (p < 0.05), which in turn did not differ from the NC group. Post-hoc tests for happy faces indicated that the NC group showed a higher bias towards happy faces than the MDD group, RMD group and the asthma group (all p < 0.05), which did not differ from each other.
Fig. 1.
Attentional bias for sad (□) and happy ( ) faces presented for 1 s for asthmatic (A), currently depressed (MDD), remitted depressed (RMD) and non-psychiatric control (NC) groups. Values are means, with standard errors represented by vertical bars.
) faces presented for 1 s for asthmatic (A), currently depressed (MDD), remitted depressed (RMD) and non-psychiatric control (NC) groups. Values are means, with standard errors represented by vertical bars.
Table 3.
Bias scores on the emotional face dot-probe task
| Group |
||||
|---|---|---|---|---|
| Facial expression | Asthma | MDD | RMD | NC |
| Sad | 1.68 (10.53)a,c | 49.50 (93.25)b | 15.42 (60.85)a,b | −29.56 (63.46)c |
| Happy | 3.82 (12.86)a | −8.42 (21.22)a | −2.10 (14.41)a | 29.65 (73.19) |
MDD, major depressive disorder group; RMD, remitted depressed group; NC, non-psychiatric healthy control group.
Values are given as mean (standard deviation).
Different superscripts within rows indicate significant group differences (p ≤ 0.05).
Because group differences on attentional bias measures do not indicate which, if any, of the groups shows a bias (see Gotlib et al. 1988), one-sample t tests were conducted comparing attentional bias scores with zero within each group. A positive bias significantly differing from zero indicates a bias towards sad/happy faces; a negative bias score indicates a bias away from sad/happy faces. A bias score that is not significantly different from zero indicates no bias for sad/happy faces. The analyses revealed that the attentional bias score for the MDD group towards sad faces was positive and significantly different from zero [t(19) = 2.37, p < 0.05] while the bias score for happy faces was negative and significantly different from zero [t(19) = −1.77, p < 0.05]. The NC group showed an opposite bias, i.e. away from sad faces [t(19) = −2.08, p < 0.05] and towards happy faces [t(19) = 1.81, p < 0.05]. For the RMD and asthma groups the attentional bias score for both sad and happy faces did not differ significantly from zero (both p > 0.05).
Emotional Stroop task
A 4 (diagnostic group) × 2 (emotion category) repeated-measures ANOVA yielded only a main effect of emotion category [F(1, 76) = 14.34, p < 0.001]. Post-hoc tests revealed that across all groups bias scores were greater for depression-specific words than for positive words, that is, all groups needed more time to name the colour of the depression-specific words than the positive words (p < 0.05) (Table 4).
Table 4.
Bias scores on the emotional Stroop task
| Group |
||||
|---|---|---|---|---|
| Category | Asthma | MDD | RMD | NC |
| sad | −5.07 (59.18) | 60.86 (180.84) | 8.53 (97.74) | 3.15 (48.91) |
| pos | −38.14 (56.18) | −26.51 (60.99) | −16.00 (67.06) | −20.59 (39.27) |
MDD, major depressive disorder group; RMD, remitted depressed group; NC, non-psychiatric healthy control group; sad, depression-specific words; pos, positive words.
Values are given as mean (standard deviation).
Discussion
By using three different cognitive tasks, the present study demonstrated depression-specific cognitive biases in currently, but also formerly, depressed participants compared with healthy control participants. Contrary to our expectations, asthma patients did not show biases to negative stimuli. We first discuss the results of each task individually, followed by an integration of the findings and their implications for research on depression-specific cognitive biases.
SRET
As expected, compared with healthy controls and asthmatics, both currently and formerly depressed participants perceived themselves in a more negative and less positive manner as quantified by better recall of negative words and being significantly slower in making a decision whether the words described themselves or not. Although less pronounced in the formerly depressed group, both depressive groups recalled less positive and more negative words they had endorsed before. No difference in the SRET task was observed between the healthy control and asthma groups, which argues against the existence of a memory bias in asthma. Most importantly, depression-specific endorsement and recall could be observed even after recovery from a depressive episode, thus replicating previous findings in currently depressed patients (Gotlib et al. 2004b). Previous studies examining decision latencies on the SRET have yielded mixed results. Gotlib et al. (2004b) could not demonstrate differences in the processing speed of emotional words between depressed patients, individuals with social phobic disorder and non-depressed individuals. Other investigators reported that clinically depressed patients were faster in evaluating negative words compared with non-depressed controls (Kuiper & MacDonald, 1982; Bradley & Mathews, 1988; Dozois & Dobson, 2001).
Emotion face dot-probe task
We also observed the expected attentional bias for sad faces in currently depressed participants and a bias away from happy faces in the currently depressed participants while, in contrast, the healthy control group selectively attended to happy faces but avoided attending to the sad faces. Most importantly, the formerly depressed participants demonstrated a comparable attentional bias for sad faces. Our results, therefore, not only replicate previous findings of an attentional bias for sad faces in current depression (Gotlib et al. 2004a), but are also in line with the few studies that have investigated biases in remitted patients (Joormann & Gotlib, 2007). Moreover, Gotlib et al. (2004a, b) could demonstrate that this attentional bias is depression specific because it was absent in participants with anxiety disorders. However, some previous studies did not find attentional biases for negative material in currently depressive patients (Mogg et al. 1995), when stimuli were presented for 500 ms instead of 1000 ms. In addition, the use of words instead of emotional faces might have led to different results (Gilboa & Gotlib, 1997; Hedlund & Rude, 1995). In this regard, interpersonal stimuli such as faces seem better suited for examining information processing, because the important function of social interaction for the improvement in depressive symptoms is well documented (Gotlib & Hammen, 1992). Rinck & Becker (2005) used a visual search task to examine depression-related biases in selective attention and found no evidence for enhanced detection of depression-related words in clinically depressed participants. However, they found that depression-related words were more distracting for the depressed than for the non-depressed participants. The asthma patients demonstrated a weaker bias to happy faces than healthy controls in the emotional dot-probe task. In addition, they did not significantly differ from the currently and formerly depressed groups. However, they allocated their attention like the healthy control group, that is, they did not look away from the happy faces as observed in the depressed persons, which argues against an attentional bias in asthma.
Emotional Stroop task
Contrary to our expectations, the four groups did not differ in their latency of colour naming. This contrasts with previous studies demonstrating a bias to negative stimuli after mood induction in currently depressed individuals (Scher et al. 2005). However, other studies were unable to find depression-specific interference in this task (Gotlib et al. 2004a). The differences might be explained by findings that both positive and negative words interfere with colour naming (Ruiz-Caballero & Bermudez, 1997), if the following word is a word with oppositional content, e.g. a positive word following a negative word.
The present study demonstrates that depression-specific cognitive patterns of information processing are not only a feature of acute depressive episodes, but are also present after recovery from depression. In contrast to most previous studies (e.g. Mogg et al. 1993; Bradley et al. 1997), this was confirmed using tasks that assess different aspects of information processing. Although less pronounced than currently depressed patients, formerly depressed persons described themselves more negatively and less positively and recalled more negative and less positive words compared with healthy participants. In addition, the formerly depressed participants differed from the healthy controls by attending selectively to sad faces while avoiding happy faces, which was comparable with the currently depressed group. However, the formerly depressed persons did not show a bias to sad faces like in other studies (Joormann & Gotlib, 2007). This difference is difficult to explain and might be related to the antidepressive medication status in some individuals of the formerly depressed group because antidepressant drug administration increases the processing of positive emotional stimuli in healthy and depressed participants (Harmer, 2008; Tranter et al. 2009). However, because a bias for negative stimuli could clearly be demonstrated in the formerly depressed group in other tests of the present study, it might be speculated that possible medication effects have different impacts on different cognitive tests, which clearly requires future research. A strength of the present study is that in contrast to most former studies all participants underwent a sound diagnostic procedure with both categorical (SCID) and dimensional (BDI, BAI, ADS, BSI) instruments to exclude any co-morbid anxiety or other mental disorder. This procedure allows attributing the observed specificity effects to current and former depression without confounding co-morbidities. However, because the currently depressive patients were recruited in a psychosomatic hospital, we cannot exclude the possibility that currently depressed out-patients might show different cognitive biases.
Our findings in the SRET and emotional dot-probe task suggest a stable depression-specific pattern of information processing and support cognitive theories of depression (Beck, 1967, 1976; Ingram, 1984; Teasdale, 1988). These theories postulate that depression-related schemata are trait-dependent and are activated by corresponding mood, which increases vulnerability for depression. Consistent with these models, the present results provide an explanation for the high risk of recurrent depressive episodes that has consistently been demonstrated (Angst, 1992; Wittchen, 2000). However, our findings of depression-specific information processing biases in formerly depressed persons cannot unambiguously be interpreted as causal factors for the development of depressive episodes. It is still possible that these biases are consequences of a preceding acute depressive episode as emphasized in the ‘scar hypothesis’ (Lewinsohn et al. 1981). In other words, it is unclear whether vulnerability for depression is caused by biased information processing being already present before the onset of a first depressive episode or whether these biases are leftover scars from experiencing the previous depressive episode. Unfortunately, such causal relationships can only be tested in large-scale prospective studies and not with a remission design. However, Joormann & Gotlib (2007) recently demonstrated that a high-risk group of never-depressed daughters of depressed mothers exhibited depression-specific information processing in the emotion face dot-probe task. This observation suggests that depression-specific information processing can be present without the experience of an initial depressive episode. Studies on neural substrates of mood-congruent biases suggest that medial and orbital-prefrontal regions may play an important role in mediating the interaction between mood and cognition in affective disorders (Elliot et al. 2002). Furthermore, it was shown that allelic variations in the promoter region of the serotonin transporter gene (5-HTTLPR) are associated with the processing of positive and negative affective material (Roiser et al. 2007; Fox et al. 2009), which might constitute neurobiological target mechanisms for pharmacological interventions (Harmer, 2008).
Consistent with cognitive theories of depression (Beck, 1967, 1976; Ingram, 1984; Teasdale, 1988), the present findings emphasize therapeutic options to prevent a relapse of depressive episodes in addition to the treatment of acute depression. They underline the importance of including interventions aimed at changing patterns of depression-specific cognitive processing such as elements from cognitive–behavioural programmes. For example, primarily cognitive therapies employing cognitive reorganization have been shown to be more effective than pharmacological or other therapeutic interventions at long-term follow-up in patients with depression (e.g. Hautzinger & de Jong-Meyer, 1996) and considerably reduced the risk of recurrent depressive episodes (e.g. Blackburn et al. 1986). Moreover, in the emotion dot-probe test formerly depressed patients demonstrated a depression-specific pattern of attending to faces, which are important cues in interpersonal interactions. This is in line with previous studies showing that interpersonal functioning remained impaired even after recovery from depression (Joiner, 2002). In addition, Gotlib & Hammen (1992) emphasized that depressed individuals’ readiness to perceive and attend to negative aspects of their social surroundings contributes to decreased levels of social support, thus leading to more depressive symptoms in a vicious circle. Interventions aimed at improving interpersonal interactions by focusing on positive and supportive social cues might thus be an important therapeutic element.
Contrary to our expectations, neither in self-description and recall nor in response times and attention to faces could we find pronounced differences between asthma patients and healthy controls. Thus, our findings argue against the presence of depression-like cognitive processing in asthma as an explanation for the high co-morbidities with depression (Zielinski & Brown, 2003). It might be speculated that such biased cognitive processing is only present in sub-groups of asthma patients, for example, those with more severe forms of the disease, which were not included in the present study. Following this lead, Serrano et al. (2006) demonstrated that in patients with a history of near-fatal asthma attacks, alexithymia is more frequent compared with patients without near-fatal asthma. Alternatively, patterns of information processing might change in the course of disease with a longer experience of asthma or a correlation could exist between the point of asthma onset and cognitive changes. For example, Miranda et al. (2004) showed that an asthma onset before the age of 12 years is associated with more asthma symptoms than a later asthma onset. Future studies are clearly required to answer these questions and should include more severe forms of asthma or different disease durations. In addition, it was interesting to examine whether cognitive biases in asthma patients recovering from depression are different than in formerly depressed participants without asthma. However, the differences among the asthma group and both depressive groups in the present study emphasize that the obtained information processing biases in the latter groups do not result per se from the experience of a disease condition, but are rather depression-specific.
Acknowledgments
This study was supported by a grant of the German Research Foundation (DFG) to A.v.L. (LE-1843/8-1). We thank Eni Becker und Mike Rinck for providing the stimuli for the emotional Stroop task, Matthias Burisch for additional statistical advice, Michael Hettich for assistance in recruiting the currently depressed participants in the Psychosomatic Hospital Bad Bramstedt, Eszter Schoell for commenting on the manuscript and all our participants.
Footnotes
Declaration of Interest
None.
References
- Allen GM, Hickie I, Gandevia SC, McKenzie DK. Impaired voluntary drive to breathe: a possible link between depression and unexplained ventilatory failure in asthmatic patients. Thorax. 1994;49:881–884. doi: 10.1136/thx.49.9.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angst J. How recurrent and predictable is depressive disorder ? In: Montgomery S, Rouillon F, editors. Long-term Treatment of Depression. Perspectives in Psychiatry. Wiley; Chichester: 1992. pp. 1–13. [Google Scholar]
- Beck AT. Depression: Clinical, Experimental, and Theoretical Aspects. Harper and Row; New York: 1967. [Google Scholar]
- Beck AT. Cognitive Therapy and the Emotional Disorders. International University Press; New York: 1976. [Google Scholar]
- Beck R, Perkins TS. Cognitive content-specificity for anxiety and depression: a meta-analysis. Cognitive Therapy and Research. 2001;25:651–663. [Google Scholar]
- Blackburn IM, Jones S, Lewin RJP. Cognitive style in depression. British Journal of Clinical Psychology. 1986;25:241–251. doi: 10.1111/j.2044-8260.1986.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Boland RJ, Keller MB. Course and outcome of depression. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Guilford Press; New York: 2002. pp. 43–60. [Google Scholar]
- Bower GH. Mood and memory. American Psychologist. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- Bradley BP, Mathews A. Memory bias in recovered clinical depressives. Cognition and Emotion. 1988;2:235–245. [Google Scholar]
- Bradley BP, Mogg K, Lee SC. Attentional biases for negative information in induced and naturally occurring dysphoria. Behaviour Research and Therapy. 1997;35:911–927. doi: 10.1016/s0005-7967(97)00053-3. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: behaviour, feeling and physiology. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford University Press; New York: 2000. pp. 242–276. [Google Scholar]
- Dozois DJA, Dobson KS. Information processing and cognitive organisation in unipolar depression: specificity and comorbidity issues. Journal of Abnormal Psychology. 2001;110:236–246. doi: 10.1037//0021-843x.110.2.236. [DOI] [PubMed] [Google Scholar]
- Dunlop DD, Lyons JS, Manheim LM, Song J, Chang RW. Arthritis and heart disease as risk factors for major depression: the role of functional limitation. Medical Care. 2004;42:502–511. doi: 10.1097/01.mlr.0000127997.51128.81. [DOI] [PubMed] [Google Scholar]
- Elliot R, Runbinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent biases in depression. Archives of General Psychiatry. 2002;59:597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Charney DS, Sahakian BJ. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. American Journal of Psychiatry. 2005;162:2171–2173. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- Fox E, Ridgewell A, Ashwin C. Looking on the bright side: biased attention and the human serotonin transporter gene. Proceedings Biological Sciences/The Royal Society Science. 2009;276:1747–1751. doi: 10.1098/rspb.2008.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke GH. Brief Symptom Inventory, German version. Hogrefe; Göttingen: 2000. [Google Scholar]
- Gilboa E, Gotlib IH. Cognitive biases and effect persistence in previously dysphoric and never-dysphoric individuals. Cognition and Emotion. 1997;11:517–538. [Google Scholar]
- Goodwin RD, Fergusson DM, Hoorwood LJ. Asthma and depressive and anxiety disorders among young persons in the community. Psychological Medicine. 2004;34:1465–1474. doi: 10.1017/s0033291704002739. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Cane D. Construct accessibility and clinical depression: a longitudinal investigation. Journal of Abnormal Psychology. 1987;96:199–204. doi: 10.1037//0021-843x.96.3.199. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Gilboa E, Sommerfeld BK. Cognitive functioning in depression: nature and origins. In: Davidson RJ, editor. Anxiety, Depression and Emotion. Oxford University Press; New York: 2000. pp. 133–163. [Google Scholar]
- Gotlib IH, Hammen CL. Psychological Aspects of Depression: Toward a Cognitive-Interpersonal Integration. Wiley; Oxford, UK: 1992. [Google Scholar]
- Gotlib IH, Kasch KL, Traill S, Joormann J, Arnow BA, Johnson SL. Coherence and specificity of information processing biases in depression and social phobia. Journal of Abnormal Psychology. 2004a;113:386–396. doi: 10.1037/0021-843X.113.3.386. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. Journal of Abnormal Psychology. 2004b;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McCann CD. Construct accessibility and depression: an examination of cognitive and affective factors. Journal of Personality and Social Psychology. 1984;47:427–439. doi: 10.1037//0022-3514.47.2.427. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, McLachlan AL, Katz AN. Biases in visual attention in depressed and nondepressed individuals. Cognition and Emotion. 1988;2:185–200. [Google Scholar]
- Hager W, Hasselhorn M. Handbook of German Affective Word Norms. Hogrefe; Göttingen, Germany: 1994. Handbuch Deurtschsprachiger Wortnormen. [Google Scholar]
- Harmer CJ. Serotonin and emotional processing: does it help explain antidepressant drug action ? Neuropharmacology. 2008;55:1023–1028. doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- Hautzinger M, Bailer M. German adaption of the Center of Epidemiologic Studies Depression Scale (CEDS) Beltz; Weinheim: 1993. Allgemeine Depressionsskala (ADS) [Google Scholar]
- Hautzinger M, Bailer M, Worall H. Beck Depression Inventory (BDI), German version. Hogrefe; Göttingen: 1994. [Google Scholar]
- Hautzinger M, de Jong-Meyer R. Two multi-centre studies on the efficacy of behavioural therapy, pharmacotherapy, and their combination in depressed patients [in German] Zeitschrift für Klinische Psychologie. 1996;25:83–160. [Google Scholar]
- Hedlund S, Rude S. Evidence of latent depressive schemas in formerly depressed individuals. Journal of Abnormal Psychology. 1995;3:517–525. doi: 10.1037//0021-843x.104.3.517. [DOI] [PubMed] [Google Scholar]
- Ingram RE. Information processing and feedback: effects of mood and information favorability on the cognitive processing of personally relevant information. Cognitive Therapy and Research. 1984;8:372–386. [Google Scholar]
- Joiner TEJ. Depression and its interpersonal context. In: Gotlib IH, Hammen CL, editors. Handbook of Depression. Guilford Press; New York: 2002. pp. 295–313. [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. Journal of Abnormal Psychology. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Keller MB, Lavori PB, Mueller TI, Endicott J, Coryell W, Hirchfeld RM, Shea T. Time to recovery, chronicity, and levels of psychopathology in major depression: a 5-year prospective follow-up of 431 subjects. Archives of General Psychiatry. 1992;49:809–816. doi: 10.1001/archpsyc.1992.01820100053010. [DOI] [PubMed] [Google Scholar]
- Kuiper NA, MacDonald MR. Self and other perception in mild depressives. Social Cognition. 1982;1:233–239. [Google Scholar]
- Kullowatz A, Kanniess F, Dahme B, Magnussen H, Ritz T. Association of depression and anxiety with health care use and quality of life in asthma patients. Respiratory Medicine. 2007;3:638–644. doi: 10.1016/j.rmed.2006.06.002. [DOI] [PubMed] [Google Scholar]
- Lang PJ. Behavioral treatment and bio-behavioral assessment: computer applications. In: Sidowski JB, Johnson JH, Williams TA, editors. Technology in Mental Health Care Delivery Systems. Ablex; Norwood, NJ: 1980. pp. 119–137. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Technical Report A-4. The Center for Research in Psychophysiology, University of Florida; Gainesville, FL: 1999. International Affective Picture System (IAPS): Instruction Manual and Affective Ratings. [Google Scholar]
- Lewinsohn PM, Steinmetz JL, Larson DW, Franklin J. Depression-related cognitions: antecedent or consequence ? Journal of Abnormal Psychology. 1981;90:213–219. doi: 10.1037//0021-843x.90.3.213. [DOI] [PubMed] [Google Scholar]
- Margraf J, Ehlers A. BAI Beck Anxiety Inventory, German version. Hogrefe; Göttingen: 2007. [Google Scholar]
- Mathews A, Ridgeway V, Williamson DA. Evidence for attention to threatening stimuli in depression. Behaviour Research and Therapy. 1996;34:695–705. doi: 10.1016/0005-7967(96)00046-0. [DOI] [PubMed] [Google Scholar]
- Matt GE, Vasquez C, Campbell WK. Mood-congruent recall of affectively toned stimuli: a meta-analytic review. Clinical Psychology. 1992;12:227–255. [Google Scholar]
- McCabe SB, Gotlib IH, Martin RA. Cognitive vulnerability for depression: deployment of attention as a function of history of depression and current mood state. Cognitive Therapy and Research. 2000;24:427–444. [Google Scholar]
- Miranda C, Busacker A, Balzar S, Trudeau J, Wenzel SE. Distinguishing severe asthma phenotypes: role of age and eosinophilic inflammation. Journal of Allergy and Clinical Immunology. 2004;113:101–108. doi: 10.1016/j.jaci.2003.10.041. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R. Attentional bias in anxiety and depression: the role of awareness. British Journal of Clinical Psychology. 1995;102:304–311. doi: 10.1111/j.2044-8260.1995.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Williams R, Mathews A. Subliminal processing of emotional information in anxiety and depression. Journal of Abnormal Psychology. 1993;102:304–311. doi: 10.1037//0021-843x.102.2.304. [DOI] [PubMed] [Google Scholar]
- Mogg K, Mathews A, Eysenck M. Attentional biases to threat in clinical anxiety states. Cognition and Emotion. 1992;6:149–159. [Google Scholar]
- Mogg K, Millar N, Bradley BP. Biases in eye movements to threatening facial expressions in generalized anxiety disorder and depressive disorder. Journal of Abnormal Psychology. 2000;109:695–704. doi: 10.1037//0021-843x.109.4.695. [DOI] [PubMed] [Google Scholar]
- Moritz S, Gläscher J, Brassen S. Investigation of mood-congruent false and true memory recognition in depression. Depression and Anxiety. 2005;21:9–17. doi: 10.1002/da.20054. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, Paykal ES. Emotional bias and inhibitory control process in mania and depression. Psychological Medicine. 1999;29:1307–1321. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- Power MJ, Dalgleish T, Claudio V, Tata P, Kentish J. The directed forgetting task: application to emotionally valent material. Journal of Affective Disorders. 2000;57:147–157. doi: 10.1016/s0165-0327(99)00084-1. [DOI] [PubMed] [Google Scholar]
- Rinck M, Becker ES. A comparison of attentional biases and memory biases in women with social phobia and major depression. Journal of Abnormal Psychology. 2005;114:62–74. doi: 10.1037/0021-843X.114.1.62. [DOI] [PubMed] [Google Scholar]
- Ritz T, Steptoe A. Emotion and pulmonary function in asthma: reactivity in the field and relationship with laboratory induction of emotion. Psychosomatic Medicine. 2000;62:808–815. doi: 10.1097/00006842-200011000-00011. [DOI] [PubMed] [Google Scholar]
- Ritz T, Steptoe A, De Wilde S, Cosat M. Emotions and stress increase respiratory resistance in asthma. Psychosomatic Medicine. 2000;62:401–412. doi: 10.1097/00006842-200005000-00014. [DOI] [PubMed] [Google Scholar]
- Roiser JP, Muller U, Clark L, Sahakian BJ. The effects of acute tryptophan depletion and serotonin transporter polymorphism on emotional processing in memory and attention. International Journal of Neuropsychopharmacology. 2007;10:449–461. doi: 10.1017/S146114570600705X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Caballero JA, Bermudez J. Anxiety and attention: is there an attentional bias for positive emotional stimuli ? Journal of General Psychology. 1997;124:194–210. doi: 10.1080/00221309709595517. [DOI] [PubMed] [Google Scholar]
- Saß H, Wittchen HU, Zaudig M. Diagnostic Statistical Manual of Mental Disorders (DSM-IV), German version. Hogrefe; Göttingen: 1996. [Google Scholar]
- Scher CD, Ingram RE, Segal ZV. Cognitive reactivity and vulnerability: empirical evaluation of construct activation and cognitive diathesis in unipolar depression. Clinical Psychology Review. 2005;25:487–510. doi: 10.1016/j.cpr.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Segal ZV, Gemar M, Williams S. Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. Journal of Abnormal Psychology. 1999;108:3–10. doi: 10.1037//0021-843x.108.1.3. [DOI] [PubMed] [Google Scholar]
- Serrano J, Plaza V, Sureda B, de Pablo J, Picado C, Bardagí S, Lamela J, Sanchis J. Alexithymia: a relevant psychological variable in near-fatal asthma. European Respiratory Journal. 2006;28:296–302. doi: 10.1183/09031936.06.00008105. [DOI] [PubMed] [Google Scholar]
- Stein MB, Cox BJ, ATO, Belik SL, Sareen J. Does co-morbid depressive illness magnify the impact of chronic physical illness ? A population-based perspective. Psychological Medicine. 2006;36:587–596. doi: 10.1017/S0033291706007239. [DOI] [PubMed] [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18:643–662. [Google Scholar]
- Teasdale JD. Cognitive vulnerability to persistent depression. Cognition and Emotion. 1988;2:247–274. [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expression in children and adults: establishing a larger stimulus set [Abstract] Journal of Cognitive Neuroscience. 2002:74. [Google Scholar]
- Tranter R, Bell D, Gutting P, Harmer C, Healy D, Anderson IM. The effect of serotonergic and noradrenergic antidepressants on face emotion processing in depressed patients. Journal of Affective Disorders. 2009 doi: 10.1016/j.jad.2009.01.028. Published online: 26 February 2009. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Dahme B. Emotions and airway resistance in asthma: study with whole body plethysmography. Psychophysiology. 2005;42:92–97. doi: 10.1111/j.1469-8986.2005.00263.x. [DOI] [PubMed] [Google Scholar]
- von Leupoldt A, Ehnes F, Dahme B. Emotion and respiratory function in asthma: a comparison of findings in everyday life and laboratory. British Journal of Health Psychology. 2006;11:185–198. doi: 10.1348/135910705X52462. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Lieb R, Pfister H, Schuster P. The waxing and waning of mental disorders: evaluating the stability of syndromes of mental disorders in the population. Comprehensive Psychiatry. 2000;41:122–32. doi: 10.1016/s0010-440x(00)80018-8. [DOI] [PubMed] [Google Scholar]
- Wittchen HU, Zaudig M, Fydrich T. Structured Clinical Interview for DSM-IV, German version. Hogrefe; Göttingen: 1997. [Google Scholar]
- Zielinski TA, Brown ES. Depression in patients with asthma. Advances in Psychosomatic Medicine. 2003;24:42–50. doi: 10.1159/000073779. [DOI] [PubMed] [Google Scholar]