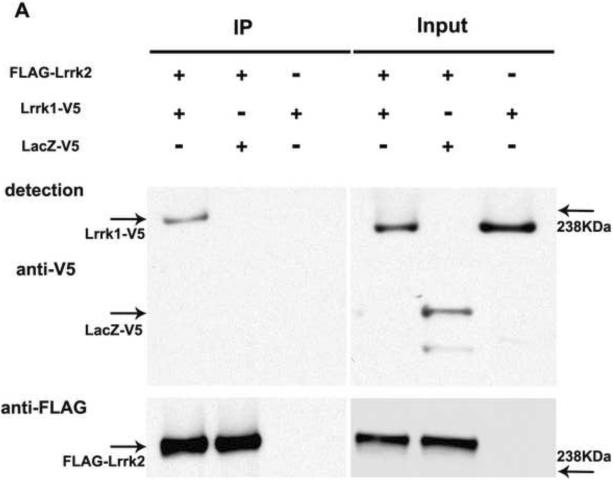
Figure 3.
Lrrk1 and Lrrk2 proteins interact in vitro: A. Co-immunoprecipitation of full length Lrrk1-V5 with FLAG-Lrrk2 in HEK293T cells. The left panel shows the presence of Lrrk1-V5 but not LacZ-V5 in the FLAG-Lrrk2 immunoprecipitate (IP). The inputs on the right panel represent the amounts of transfected proteins introduced in the immunoprecipitation assay.B. Co-precipitation of Lrrk2-V5 with FLAG-Lrrk1. Successful co-pull-down of Lrrk2-V5 occurs only in the presence of FLAG-Lrrk1 (IP left; Inputs right). A stronger detection signal was observed when Lrrk2 was used to pull-down Lrrk1 than vice versa, which may indicate a higher affinity of Lrrk2 for Lrrk1, whereas Lrrk1 might preferentially engage in interactions with other proteins. However as co-immunoprecipitation assays do not provide a reliable quantitative assessment of binding affinities this warrants further investigation.