Abstract
Targeted antibody-based therapy has been successfully used to treat cancers. Recent studies have shown that tumor cells treated with antibodies specific forβ2-microglobulin (β2M) or major histocompatibility complex (MHC) class I undergo apoptosis in vitro and in vivo (mouse models). Antibodies against β2M or MHC class I induce tumor cell apoptosis via 1) recruiting MHC class I molecules to lipid rafts and activating Lyn and the signal transducing enzyme phospholipase C-γ2–dependent JNK signaling pathway and 2) expelling IL-6 and IGF-1 receptors out of lipid rafts and inhibiting the growth and survival factor-induced activation of PI3K/Akt and ERK pathways. Consequently, mitochondrial integrity is compromised and the caspase-9–dependent cascade is activated in treated tumor cells. However, although β2M and MHC class I are expressed on normal hematopoietic cells, which is a potential safety concern, the mAbs were selective to tumor cells and did not damage normal cells in vitro and in human-like mouse models. These findings suggest that targeting β2M or MHC class I by antibodies or other agents offers a potential therapeutic approach for β2M/MHC class I-expressing malignancies.
Keywords: β2M, MHC class I, monoclonal antibodies, tumor cell apoptosis, signaling pathways
Introduction
MHC class I molecules consist of a 45-kDa α-chain that contains domains α1, α2, and Ig-like domain α3, and an 11.6-kDa light chain called β2-microglobulin (β2M). The α1 and α2 domains of the α-chain are polymorphic. Their polymorphisms frequently occur in three hypervariable regions that form the antigen-binding cleft or peptide-binding region, which is recognized by the T-cell receptor on CD8+ T lymphocytes. Domain α3 contains a conserved seven-amino acid loop that binds with CD8 molecules 1, 2. β2M is a non-glycosylated polypeptide composed of 100 amino acids. Its best characterized function is to interact with and stabilize the tertiary structure of the α-chain 3. Because it is non-covalently associated with the α-chain, it can be exchanged with the circulating form of β2M, which is present at low levels in serum, urine, and other body fluids under physiological conditions 4.
β2M/MHC class I molecules are found on almost all normal nucleated cells and on most tumor cells, although the levels of expression may differ among different cells 5. While some solid tumors express a low density of β2M/MHC class I molecules on their surface 6, 7 to escape host immune surveillance 8, 9, overexpression of β2M/MHC class I molecules has also been reported on other tumors, including hematological malignancies 10. Thus, these molecules are potential targets of antibody-based therapy for β2M/MHC class I-positive tumors 11, 12.
MHC class I as signaling molecules
MHC class I molecules are important signal-transducing molecules involved in the finely tuned regulation of immune responses. Ligation of MHC class I molecules on T and B cells by immobilized antibodies or secondary cross-linking triggers signal transduction, which is involved in responses ranging from anergy and apoptosis to cell proliferation and IL-2 production 13–17. Cross-linking MHC class I activates several intracellular signaling pathways, including: 1) phosphorylation of tyrosine kinases leading to a rise in the intracellular free calcium concentration, 2) activation of the JAK/STAT pathway resulting in STAT3 activation, and 3) upregulation of PI3K leading to JNK activation 13–17. However, it is yet unclear as to which part of MHC class I molecules transmits the signals. The cytoplasmic domain of MHC class I α-chain has a tyrosine 320 residue, which can be phosphorylated and forms a signaling motif. However, previous studies have shown that deletion of all but the four proximal amino acids from the cytoplasmic tail does not alter their signal transduction capabilities 18, and truncated molecules are still able to synergize with CD3, CD2, or CD28 to initiate IL-2 production 19, 20. On the other hand, others have shown that MHC class I molecules are physically associated with some hormone or growth factor receptors, such as insulin receptor, insulin-like growth factor (IGF) receptor, epidermal growth factor receptor, IL-2 receptor, IL-4 receptor, and glucagon receptors on cell surfaces 21–26, suggesting that MHC class I-induced signaling may be transmitted through these receptors. Taken together, these findings indicate that, in addition to antigen presentation, MHC class I molecules or their components play an important role in the regulation of immune responses via MHC class I-mediated signaling.
MHC class I as an inducer of cell apoptosis
In the past decades, antibodies targeting surface MHC class I molecules on various cell types have been generated and investigated. Genestier reported that two monoclonal antibodies (mAbs; mouse mAb90 and rat YTH862), which bind epitopes of theα1 domain of the α-chain, induce apoptosis in activated, but not resting, T lymphocytes 27 and CD40-activated B lymphocytes 28. Another mAb, a rat mAb RE2 that bound with α2 domain of the α-chain, induced apoptosis in activated murine lymphocytes that involved caspase cascade and PI3K activation 29. Others reported that a murine mAb (5H7) specific for the α3 domain, but not other mAbs (TI2599, α3 domain-specific and W6/32, binding with both α2 and α3), could induce growth inhibition and apoptosis of B-cell–derived tumor cell lines 30, 31. However, secondary cross-linking of the mAb was required, because only plastic-immobilized but not soluble 5H7 mAb were able to kill the cells 30, 31.
Antibodies against surface β2M are therapeutic against hematological malignancies
Elevated levels of circulating β2M, present in hematological malignancies such as multiple myeloma 32, lymphomas 33, and leukemias 34, are one of important predictive factors in these cancer patients for indication of aggressive diseases and poor survival prognosis 35. Previous studies have shown that release of β2M into surrounding tissues may contribute to the induction of bone absorption in patients with myeloma 36 by inducing osteoclast formation. In addition, β2M may induce osteoblast secretion of IL-6 37 and promote fibroblast production of MMP-1 38, both of which support tumor growth and bone destruction. These findings indicate that targeting β2M may help control tumor growth and bone destruction in these malignancies.
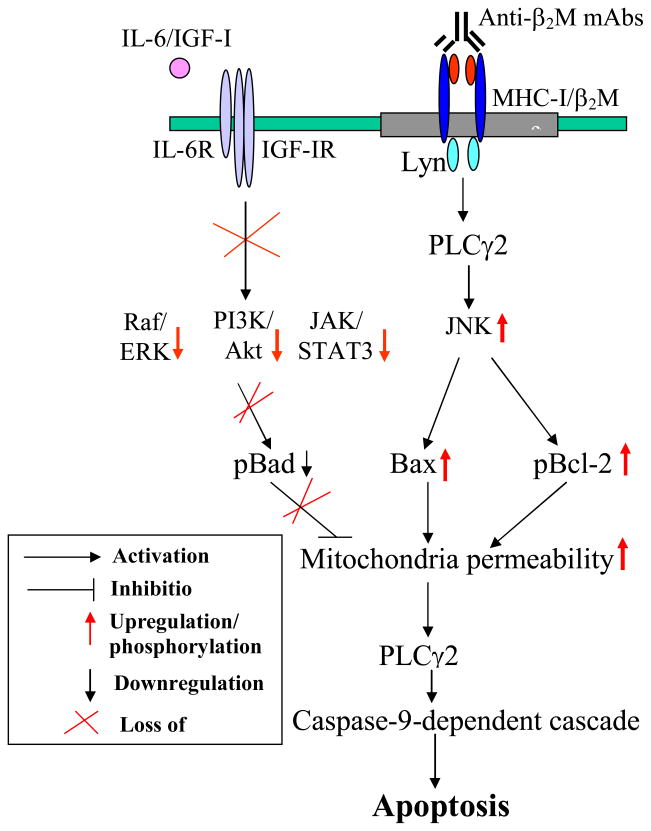
Our recent studies showed that anti-β2M mAbs induce programmed death of hematological malignant cells 39. We used a commercially available anti-β2M mAb B2 and mAbs D1 and E6 made in our own laboratory and showed that these mAbs exhibited potent in vitro tumoricidal activity against all 14 of β2M/MHC class I-expressing multiple myeloma, Burkitt lymphoma, mantle cell lymphoma, T-cell and myelogenous leukemia cell lines, and primary tumor cells isolated from patients with myeloma that were tested. Tumor cell death occurred rapidly, without the need for exogenous immunological effector mechanisms or secondary cross-linking. Although the expression of β2M on normal hematopoietic cells is a potential safety concern, the mAbs seemed to be selective to tumor-transformed cells and did not induce apoptosis of normal cells, including T and B lymphocytes and CD34+ bone marrow stem cells. Furthermore, the mAbs were able to selectively kill myeloma cells without damaging normal stromal cells in their cocultures. After binding to cell surface, the mAbs mediated internalization and down-modulation of surface β2M and MHC class I molecules. The mAbs induced cell death via upregulating Bad and Bax protein expression, inducing phosphorylation of Bcl-2, and decreasing phosphorylation of Bad, all of which compromised mitochondrial integrity, leading to cytochrome c release into cytosol and activation of the caspase-9-dependent cascade. Inhibitors to pan-caspases or caspase-9, but not to caspase-8, prevented anti-β2M mAb-mediated tumor cell apoptosis. Moreover, knockdown of surface β2M by β2M-specific siRNAs decreased the expression of MHC class I molecules on tumor cells, and consequently abrogated apoptosis of tumor cells induced by the mAbs. Furthermore, our studies showed that anti-β2M mAbs are also active and therapeutic in vivo. After subcutaneous or intraperitoneal injections, the mAbs significantly reduced tumor burdens and retarded tumor growth in xenografted SCID mice with human myeloma, lymphoma, and leukemia cell lines and in SCID-hu mice inoculated with primary myeloma cells isolated from patients. These findings indicate that use of anti-β2M mAbs are a potential novel therapeutic approach to treating hematological malignancies that express surface β2M/MHC class I molecules.
For future clinical application of the mAbs as a therapeutic agent, a major concern is whether anti-β2M mAbs will be therapeutic and safe to treat cancer patients in whom every tissue expresses low densities of MHC class I molecules and elevated levels of soluble β2M are present. To address this concern, we recently developed and used a myeloma-HLA-A2-transgenic NOD/SCID mouse model 40. The mice are transgenic for HLA-A2 α-chain but not human β2M. However, with established myeloma, all murine tissues express human HLA-A2 and β2M, and high levels of circulating human β2M, which are seen in most of myeloma patients, were detected, indicating that myeloma-derived human β2M form mature MHC class I molecules with the HLA-A2 α-chain on murine cells. Moreover, the human MHC class I molecules on murine cells are functional. We found that anti-β2M mAbs effectively suppress myeloma growth in these mice, activate caspase-9 and -3, and induce myeloma cell apoptosis in vivo. Although the mAbs can be detected on different organs including the heart, lung, spleen, liver, and kidney, no tissue damage or cell apoptosis or associated caspase activation was observed in the mice 40. Moreover, treatment with anti-β2M mAbs did not change the body weight of the mice or impair the implanted human bones in SCID-hu mice 39.
We also evaluated whether the therapeutic effects of the mAbs would be compromised by β2M/MHC class I-expressing normal cells using in vitro cocultures, which contains myeloma cells and 5-fold more numbers of β2M/MHC class I-expressing normal peripheral blood mononuclear cells (PBMCs). We found that anti-β2M mAbs killed myeloma cells with the same efficiency irrespectively whether they were surrounded and outnumbered by normal cells expressing human β2M/MHC class I. In addition, although the bone marrow stromal cells such as osteoclasts promote tumor growth and protect myeloma cells from apoptosis in vitro, mAb-induced myeloma cell apoptosis was not protected in the cocultures with osteoclasts 39. Furthermore, addition of higher molar concentrations of solubleβ2M (50–100 μg/ml), which is 3- to 10-fold higher that those in patients with myeloma, in the culture of myeloma cells did not abrogate the apoptosis-inducing effects of the mAbs on myeloma 39. These findings strongly suggest that anti-β2M mAbs may be efficacious and that their therapeutic effects in cancer patients may not be compromised by tissue-expressed and soluble β2M.
We have carefully examined the potential toxicity of anti-β2M mAbs on normal hematopoietic cells. Normal PBMCs, resting and activated CD3+ T cells and CD19+ B cells, CD16+ NK cells, and bone marrow CD34+ stem cells were treated with the mAbs and were found to be resistant to the mAb-induced apoptosis 39. However, Smith et al. reported that treatment with 5H7, a mAb specific for the α3 domain of MHC class I molecules, caused suppression of T cell-mediated cellular immunity in vivo 31. To investigate the possibility that anti-β2M mAbs would trigger the potential immunological consequences by binding to and blocking MHC class I on normal cells, we examined whether the mAbs could induce NK cells to kill normal cells coated with the mAbs in vitro and in vivo. The results showed that NK cells did not lyse PBMCs pretreated with or without the mAb or IgG1. However, these NK cells efficiently killed K562 cells. Furthermore, no cell apoptosis or tissue damage were observed in the human bone marrow tissue, after immunohistochemical examination of the human marrow cells from SCID-hu mice in which anti-β2M mAb and subsequently purified human NK cells were injected directly into the implanted human bones 39. Collectively, these in vitro and in vivo toxicity data using humanized mouse models provide strong evidence that targeting β2M using monoclonal antibodies will have limited direct toxicity if this approach were translated into a therapeutic strategy despite the ubiquitous expression of β2M and class I MHC on the majority of tissues. However, there is a possibility that by blocking β2M or MHC class I molecules, these mAbs could inhibit MHC class I antigen presentation and induce immunosuppression. This possibility needs to be investigated.
Similar results are reported from other mAb-based therapy for cancers, such as anti-epidermal growth factor receptor (EGFR) mAbs, including cetuximab, panitummab, and matuzumab 41, 42. Anti-EGFR mAbs effectively eradicate tumor cells and are currently used in the treatment of several solid cancers 41. Like human β2M/MHC class I molecules, EGFR is widely expressed by normal cells of epithelial, mesenchymal, and neuronal origin 41, and anti-EGFR mAbs still have low toxicity on these normal cells. The reasons might be partially due to overexpression of the antigens in tumor cells 39, 41, since our and other’s studies showed that myeloma cells express significantly higher levels of surface β2M than normal cells 39, 43. In addition, the ability of mAbs to cross-link β2M/MHC class I molecules and mAb-mediated intracellular signaling pathways might be different on normal cells, which may have contributed to normal cell-resistance to the mAb-induced apoptosis. Indeed, our studies showed that the mAbs did not recruit MHC class I molecules to lipid raft and activate the downstream apoptotic signaling pathways in normal B cells 39. Nevertheless, as this is an important issue for future clinical application, further study will be needed to examine the potential toxicity in non-human primates using humanized mAbs against β2M or MHC class I molecules.
In addition to naturally generated mAbs, Sekimoto and colleagues recently developed a recombinant single-chain Fv diabody (2D7-DB) specific to the α2 domain of HLA-A and showed that 2D7-DB specifically induced myeloma cell death in the bone marrow environment 44. The diabody rapidly induced Rho-mediated actin aggregation in a caspase-independent death pathway in myeloma cells without damaging normal bone marrow cells. Furthermore, the diabody synergized with interferon-γ and chemotherapy drugs melphalan or bortezomib in killing myeloma cells 44. Importantly, the single-chain diabody lacks Fc fragment so that it cannot bind or activate immune effector molecules or cells such as complements or NK cells, and will not be able to damage normal cells via mediating CDC or ADCC. Thus, targeting surface β2M/MHC class I molecules may be a novel therapeutic approach against hematological malignancies.
Antibodies against surface β2M induce apoptosis in solid tumors
Overexpression of surface β2M/MHC class I was also detected in some types of solid tumors, including prostate cancer 43, renal cell carcinoma 45, 46, gastrointestinal 47, lung, and breast cancers 48, 49. Previous studies have shown that increased β2M expression linked to increased tumor growth and enhanced migration and invasion of breast cancer, lung cancer and renal cell carcinoma 45, and surface β2M can be regarded as a signaling and growth-promoting factor for prostate cancer and cancer-associated bone metastasis 43,50. Interestingly, both polyclonal antibodies and mAbs specific for surface β2M had strong tumoricidal activity in prostate cancer and renal cell carcinoma 51. Treatment of several renal cell carcinoma lines with anti-β2M antibodies strongly suppressed the growth and induced apoptosis of these cells in vitro in a dose- and time-dependent manner 52. In addition, Huang and colleagues demonstrated that the downstream signaling pathways of surface β2M/MHC class I directly regulate the expression of androgen receptor, a prostate cancer survival factor, and its target gene prostate-specific antigen in prostate cancer 53. Anti-β2M antibodies inhibited the growth and induced apoptosis of prostate cancer cells via interrupting β2M-mediated androgen receptor and prostate-specific antigen expression 53. These studies indicate that targeting surface β2M may be a novel and promising therapeutic agent for the treatment of β2M/MHC class I-positive solid tumors as well.
Ligation of β2M/MHC class I with antibodies activates apoptotic pathways via lipid rafts in tumor cells
Studies have shown that β2M/MHC class I molecules possess the ability to regulate receptor-mediated transmembrane signal transduction 11, 54, 55. Ligation of β2M/MHC class I molecules with antibodies induced clustering, capping, and internalization of MHC class I molecules and activation of several intracellular signaling pathways in normal cells 13–17. As a consequence, phosphorylation of tyrosine kinases led to a rise in the concentration of intracellular free calcium, activation of JAK/STAT3 pathway 13, and upregulated PI3K and JNK activities 17. In tumor cells, ligation of MHC class I molecules with specific antibodies induced apoptotic signaling pathways via stimulating phosphorylation of kinases ASK, MLK3, and MEKK1; upregulating MKK4/7 activity; and activating JNK. In vitro pretreatment of cells with JNK inhibitor could completely abrogate anti-β2M mAb-induced apoptosis, and injection of JNK inhibitor in vivo reduced mAb-mediated tumoricidal effects on established tumor cells in SCID mouse models. Moreover, anti-β2M mAbs disrupted IL-6– or IGF-1–stimulated Ras/Raf/ERK1/2 and PI3K/Akt signaling pathways in myeloma cells 39, 55. Different from Fas/Fas ligand-induced caspase-8 activation, treatment of tumor cells with anti-β2M mAbs compromised mitochondrial integrity and activated caspase-9-dependent apoptotic cascades 39, 56, 57.
Our studies have also implicated lipid rafts as an important mediator for anti-β2M mAb-induced apoptosis in tumor cells. Lipid rafts, cholesterol- and glycosphingolipid-enriched dynamic patches in the plasma membrane of cells, organize plasma membrane into functional units. Accumulating evidence has suggested that lipid rafts act as platforms to transduce signals into cells for various functions 58, and are involved in anti-MHC class II and anti-CD20 mAb-induced apoptosis of tumor cells 59, 60. Under physiological conditions, some MHC class II molecules are found in lipid rafts, and more of the molecules are located within lipid rafts after treatment with anti-MHC class II mAbs. Such relocalization of MHC class II molecules seems to be critical for anti-HLA-DR mAb-mediated tumor cell apoptosis. Nagy and Mooney showed that MHC class II-specific mAb mediated signaling events and pathological changes on tumor cells by recruiting MHC class II molecules into lipid rafts and localizing actin and PKC to the rafts, which in turn activated PKC and its potential targets, leading to tumor cell apoptosis 61. Different from MHC class II, MHC class I molecules are present outside lipid rafts under physiological situation. However, after treatment with anti-β2M mAbs, MHC class I molecules on myeloma cells were recruited into lipid rafts, leading to activation of src family tyrosine kinase Lyn and PLC-γ2 39. In contrast, neither MHC class I molecules nor Lyn were associated with the lipid rafts in normal B cells treated with or without anti-β2M mAbs, which could partially explain the selectivity of anti-β2M mAbs against malignant but not normal cells. In addition, anti-β2M mAbs also affected the raft distribution of growth factor receptors in tumor cells. It has been shown that growth and survival factors such as IL-6 and IGF-1 stimulate growth signaling and confer protection against chemotherapy drug-induced apoptosis due to translocation of their receptors into lipid rafts 62. We observed that anti-β2M mAb binding to surface β2M/MHC class I molecules not only recruits MHC class I molecules into lipid rafts, but also expels growth factor (IL-6 and IGF-1) receptors out of lipid rafts 56, thereby disrupting their signaling pathways. Myeloma cells treated with methyl-β-cyclodextrin, an agent that disrupts the structure of lipid rafts in cell membrane, were no longer sensitive to anti-β2M mAb-induced apoptosis. These observations further confirm the association and importance of MHC class I and lipid rafts in anti-β2M mAb-induced apoptosis in myeloma cells.
Summary and conclusions
Taken together, antibodies against β2M/MHC class I molecules exhibited tumoricidal activity against several tumor cell lines through surface β2M/MHC class I molecules and their associated pro-apoptotic signaling pathways in preclinical studies. Targeting β2M/MHC class I molecules has significant advantages in the treatment of β2M/MHC class I-expressing tumors, because these mAbs have remarkably strong tumoricidal activities and are effective at killing all β2M/MHC class I-expressing hematological malignant cells examined without the need for exogenous immunological effector mechanisms. The mAbs are able to kill chemotherapy-refractory myeloma in vitro and more importantly, lead to tumor regression in xenograft mouse models of myeloma and other hematological cancers, without damaging normal cells and tissues. Further, their therapeutic efficacy was not counteracted by the high concentrations of soluble β2M and tissue-expressing β2M/MHC class I. Although, the animal models reviewed here suggest that normal cells expressing MHC class I and soluble β2M do not significantly impair the efficacy of anti-β2M antibodies, this could still limit application of this therapeutic stragegy in patients that have high levels of circulating β2M. The potential for immunosuppression due to blockade of surface β2M or MHC class I molecules requires further study prior to clinical translation of this approach.
Figure 1.
Anti-β2M mAb-mediated apoptotic signaling pathways in myeloma cells.
Acknowledgments
National Cancer Institute R01 CA96569, R01 CA103978, and R01 CA138402, the Leukemia and Lymphoma Society Translational Research Grants, Multiple Myeloma Research Foundation, Commonwealth Foundation for Cancer Research, and funds from the University Cancer Foundation and the Center for Targeted Therapy of The University of Texas M. D. Anderson Cancer Center (to Q. Yi); and National Cancer Institute K99/R00 CA137158, International Myeloma Foundation, Lymphoma Research Foundation, and American Society of Hematology (to J. Yang).
Footnotes
Conflict of Interest Disclosures
References
- 1.Yewdell JW, Reits E, Neefjes J. Making sense of mass destruction: quantitating MHC class I antigen presentation. Nat Rev Immunol. 2003;3:952–961. doi: 10.1038/nri1250. [DOI] [PubMed] [Google Scholar]
- 2.Buus S. MHC restricted antigen presentation and T cell recognition. Dan Med Bull. 1994;41:345–358. [PubMed] [Google Scholar]
- 3.Bjorkman PJ, Burmeister WP. Structures of two classes of MHC molecules elucidated: crucial differences and similarities. Curr Opin Struct Biol. 1994;4:852–856. doi: 10.1016/0959-440x(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 4.Strominger JL. Human histocompatibility proteins. Immunol Rev. 2002;185:69–77. doi: 10.1034/j.1600-065x.2002.18508.x. [DOI] [PubMed] [Google Scholar]
- 5.Natarajan K, Li H, Mariuzza RA, et al. MHC class I molecules, structure and function. Rev Immunogenet. 1999;1:32–146. [PubMed] [Google Scholar]
- 6.Scupoli MT, Sartoris S, Tosi G, et al. Expression of MHC class I and class II antigens in pancreatic adenocarcinomas. Tissue Antigens. 1996;48:301–311. doi: 10.1111/j.1399-0039.1996.tb02649.x. [DOI] [PubMed] [Google Scholar]
- 7.Algarra I, Collado A, Garrido F. Altered MHC class I antigens in tumors. Int J Clin Lab Res. 1997;27:95–102. doi: 10.1007/BF02912442. [DOI] [PubMed] [Google Scholar]
- 8.Seliger B, Harders C, Wollscheid U, et al. Suppression of MHC class I antigens in oncogenic transformants: association with decreased recognition by cytotoxic T lymphocytes. Exp Hematol. 1996;24:1275–1279. [PubMed] [Google Scholar]
- 9.Bubenik J. MHC class I down regulation, tumour escape from immune surveillance and design of therapeutic strategies. Folia Biol (Praha) 2005;51:1–2. [PubMed] [Google Scholar]
- 10.Musarurwa C, Matarira HT. Beta-2-microglobulin in multiple myeloma. Cent Afr J Med. 2004;50:19–20. [PubMed] [Google Scholar]
- 11.Fishman D, Elhyany S, Segal S. Non-immune functions of MHC class I glycoproteins in normal and malignant cells. Folia Biol (Praha) 2004;50:35–42. [PubMed] [Google Scholar]
- 12.Tanaka K, Kasahara M. The MHC class I ligand-generating system: roles of immunoproteasomes and the interferon-gamma-inducible proteasome activator PA28. Immunol Rev. 1998;163:161–176. doi: 10.1111/j.1600-065x.1998.tb01195.x. [DOI] [PubMed] [Google Scholar]
- 13.Skov S, Nielsen M, Bregenholt S, et al. Activation of Stat-3 is involved in the induction of apoptosis after ligation of major histocompatibility complex class I molecules on human Jurkat T cells. Blood. 1998;91:3566–3573. [PubMed] [Google Scholar]
- 14.Skov S, Bregenholt S, Claesson MH. MHC class I ligation of human T cells activates the ZAP70 and p56lck tyrosine kinases, leads to an alternative phenotype of the TCR/CD3 zeta-chain, and induces apoptosis. J Immunol. 1997;158:3189–3196. [PubMed] [Google Scholar]
- 15.Ruhwald M, Pedersen AE, Claesson MH. MHC class I cross-talk with CD2 and CD28 induces specific intracellular signalling and leads to growth retardation and apoptosis via a p56(lck)-dependent mechanism. Exp Clin Immunogenet. 1999;16:199–211. doi: 10.1159/000019112. [DOI] [PubMed] [Google Scholar]
- 16.Skov S, Odum N, Claesson MH. MHC class I signaling in T cells leads to tyrosine kinase activity and PLC-gamma 1 phosphorylation. J Immunol. 1995;154:1167–1176. [PubMed] [Google Scholar]
- 17.Lamberth K, Claesson MH. Ligation of major histocompatibility complex class I antigens (MHC-I) prevents apoptosis induced by Fas or SAPK/JNK activation in T-lymphoma cells. Tissue Antigens. 2001;58:171–180. doi: 10.1034/j.1399-0039.2001.580305.x. [DOI] [PubMed] [Google Scholar]
- 18.Gur H, Geppert TD, Wacholtz MC, et al. The cytoplasmic and the transmembrane domains are not sufficient for class I MHC signal transduction. Cell Immunol. 1999;191:105–116. doi: 10.1006/cimm.1998.1417. [DOI] [PubMed] [Google Scholar]
- 19.Gur H, el-Zaatari F, Geppert TD, et al. Analysis of T cell signaling by class I MHC molecules: the cytoplasmic domain is not required for signal transduction. J Exp Med. 1990;72:1267–1270. doi: 10.1084/jem.172.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stagsted J, Reaven GM, Hansen T, et al. Regulation of insulin receptor functions by a peptide derived from a major histocompatibility complex class I antigen. Cell. 1990;62:297–307. doi: 10.1016/0092-8674(90)90367-n. [DOI] [PubMed] [Google Scholar]
- 21.Stagsted J, Hansen T, Roth RA, et al. Correlation between insulin receptor occupancy and tyrosine kinase activity at low insulin concentrations and effect of major histocompatibility complex class I-derived peptide. J Pharmacol Exp Ther. 1993;267:997–1001. [PubMed] [Google Scholar]
- 22.Stagsted J, Olsson L, Holman GD, et al. Inhibition of internalization of glucose transporters and IGF-II receptors. Mechanism of action of MHC class I-derived peptides which augment the insulin response in rat adipose cells. J Biol Chem. 1993;268:22809–22813. [PubMed] [Google Scholar]
- 23.Centrella M, McCarthy TL, Canalis E. Beta 2-microglobulin enhances insulin-like growth factor I receptor levels and synthesis in bone cell cultures. J Biol Chem. 1989;264:18268–18271. [PubMed] [Google Scholar]
- 24.Schreiber AB, Schlessinger J, Edidin M. Interaction between major histocompatibility complex antigens and epidermal growth factor receptors on human cells. J Cell Biol. 1984;98:725–731. doi: 10.1083/jcb.98.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stagsted J, Ziebe S, Satoh S, et al. Insulinomimetic effect on glucose transport by epidermal growth factor when combined with a major histocompatibility complex class I-derived peptide. J Biol Chem. 1993;268:1770–1774. [PubMed] [Google Scholar]
- 26.Sharon M, Gnarra JR, Baniyash M, Leonard WJ. Possible association between IL-2 receptors and class I HLA molecules on T cells. J Immunol. 1988;141:3512–3515. [PubMed] [Google Scholar]
- 27.Genestier L, Paillot R, Bonnefoy-Berard N, et al. Fas-independent apoptosis of activated T cells induced by antibodies to the HLA class I alpha1 domain. Blood. 1997;90:3629–3639. [PubMed] [Google Scholar]
- 28.Genestier L, Meffre G, Garrone P, et al. Antibodies to HLA class I alpha1 domain trigger apoptosis of CD40-activated human B lymphocytes. Blood. 1997;90:726–735. [PubMed] [Google Scholar]
- 29.Matsuoka S, Tsurui H, Abe M, et al. A monoclonal antibody to the alpha2 domain of murine major histocompatibility complex class I that specifically kills activated lymphocytes and blocks liver damage in the concanavalin A hepatitis model. J Exp Med. 2003;198:497–503. doi: 10.1084/jem.20021301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woodle ES, Smith DM, Zhou N. Class I MHC mediates programmed cell death in human lymphoid cells. Transplantation. 1997;64:140–146. doi: 10.1097/00007890-199707150-00024. [DOI] [PubMed] [Google Scholar]
- 31.Smith DM, Bluestone JA, Jeyarajah DR, et al. Inhibition of T cell activation by a monoclonal antibody reactive against the alpha 3 domain of human MHC class I molecules. J Immunol. 1994;153:1054–1067. [PubMed] [Google Scholar]
- 32.Bataille R, Durie BG, Grenier J. Serum beta2 microglobulin and survival duration in multiple myeloma: a simple reliable marker for staging. Br J Haematol. 1983;55:439–447. doi: 10.1111/j.1365-2141.1983.tb02158.x. [DOI] [PubMed] [Google Scholar]
- 33.Cooper EH, Plesner T. Beta-2-microglobulin review: its relevance in clinical oncology. Med Pediatr Oncol. 1980;8:323–334. doi: 10.1002/mpo.2950080403. [DOI] [PubMed] [Google Scholar]
- 34.Molica S, Levato D, Cascavilla N, et al. Clinico-prognostic implications of simultaneous increased serum levels of soluble CD23 and beta2-microglobulin in B-cell chronic lymphocytic leukemia. Eur J Haematol. 1999;62:117–122. doi: 10.1111/j.1600-0609.1999.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 35.Rajkumar SV, Fonseca R, Lacy MQ, et al. A. Beta2-microglobulin and bone marrow plasma cell involvement predict complete responders among patients undergoing blood cell transplantation for myeloma. Bone Marrow Transplant. 1999;23:1261–1266. doi: 10.1038/sj.bmt.1701787. [DOI] [PubMed] [Google Scholar]
- 36.Sato Y, Kuno H, Kaji M, et al. Serum beta2-microglobulin reflects increased bone resorption in immobilized stroke patients. Am J Phys Med Rehabil. 2001;80:19–24. doi: 10.1097/00002060-200101000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Balint E, Marshall CF, Sprague SM. Role of interleukin-6 in beta2-microglobulin-induced bone mineral dissolution. Kidney Int. 2000;57:1599–1607. doi: 10.1046/j.1523-1755.2000.00004.x. [DOI] [PubMed] [Google Scholar]
- 38.Ohashi K. Pathogenesis of beta2-microglobulin amyloidosis. Pathol Int. 2001;51:1–10. doi: 10.1046/j.1440-1827.2001.01156.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Qian J, Wezeman M, et al. Targeting beta(2)-microglobulin for induction of tumor apoptosis in human hematological malignancies. Cancer Cell. 2006;10:295–307. doi: 10.1016/j.ccr.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 40.Yang J, Cao Y, Hong S, et al. Human like-mouse models for testing the efficacy and safety of anti-beta2-microblobulin monoclonal antibodies to treat myeloma. Clin Cancer Res. 2009;3:951–959. doi: 10.1158/1078-0432.CCR-08-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendelsohn J, Baselga J. The EGF receptor family as targets for cancer therapy. Oncogene. 2000;19:6550–6565. doi: 10.1038/sj.onc.1204082. [DOI] [PubMed] [Google Scholar]
- 42.Kovacs MJ, Reece DE, Marcellus D, Meyer RM, Mathews S, Dong RP, Eisenhauer E. A phase II study of ZD6474 (Zactima, a selective inhibitor of VEGFR and EGFR tyrosine kinase in patients with relapsed multiple myeloma--NCIC CTG IND.145. Invest New Drugs. 2006;24:529–535. doi: 10.1007/s10637-006-9022-7. [DOI] [PubMed] [Google Scholar]
- 43.Huang WC, Wu D, Xie Z, et al. beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–9116. doi: 10.1158/0008-5472.CAN-06-1996. [DOI] [PubMed] [Google Scholar]
- 44.Sekimoto E, Ozaki S, Ohshima T, et al. A single-chain Fv diabody against human leukocyte antigen-A molecules specifically induces myeloma cell death in the bone marrow environment. Cancer Res. 2007;67:1184–1192. doi: 10.1158/0008-5472.CAN-06-2236. [DOI] [PubMed] [Google Scholar]
- 45.Rasmuson T, Grankvist K, Ljungberg B. Serum beta 2-microglobulin and prognosis of patients with renal cell carcinoma. Acta Oncol. 1996;35:479–482. doi: 10.3109/02841869609109926. [DOI] [PubMed] [Google Scholar]
- 46.Selli C, Cozzolino F, Carini M, et al. Serum beta 2 microglobulin levels in patients with renal cell carcinoma. Urol Res. 1984;12:261–263. doi: 10.1007/BF00256152. [DOI] [PubMed] [Google Scholar]
- 47.Staab HJ, Anderer FA, Hiesche K, et al. Is serum beta 2-microglobulin a tumor marker in gastrointestinal cancer? Clin Chim Acta. 1980;106:309–317. doi: 10.1016/0009-8981(80)90315-0. [DOI] [PubMed] [Google Scholar]
- 48.Chen HL, Gabrilovich D, Virmani A, et al. Structural and functional analysis of beta2 microglobulin abnormalities in human lung and breast cancer. Int J Cancer. 1996;67:756–763. doi: 10.1002/(SICI)1097-0215(19960917)67:6<756::AID-IJC2>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 49.Papaioannou D, Geggie P, Klassen J. Study of serum beta-2 microglobulin levels in breast cancer patients. Clin Chim Acta. 1979;99:37–41. doi: 10.1016/0009-8981(79)90137-2. [DOI] [PubMed] [Google Scholar]
- 50.Nomura T, Huang WC, Zhau HE, et al. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12:7294–7305. doi: 10.1158/1078-0432.CCR-06-2060. [DOI] [PubMed] [Google Scholar]
- 51.Nomura T, Huang WC, Seo S, et al. Targeting beta2-microglobulin mediated signaling as a novel therapeutic approach for human renal cell carcinoma. J Urol. 2007;178:292–300. doi: 10.1016/j.juro.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 52.Freeman MR. Beta2 microglobulin: a surprising therapeutic target for prostate cancer and renal cell carcinoma. J Urol. 2007;178:10–11. doi: 10.1016/j.juro.2007.03.203. [DOI] [PubMed] [Google Scholar]
- 53.Huang WC, Havel JJ, Zhau HE, et al. Beta2-microglobulin signaling blockade inhibited androgen receptor axis and caused apoptosis in human prostate cancer cells. Clin Cancer Res. 2008;14:5341–5347. doi: 10.1158/1078-0432.CCR-08-0793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skov S. Intracellular signal transduction mediated by ligation of MHC class I molecules. Tissue Antigens. 1998;51:215–223. [PubMed] [Google Scholar]
- 55.Tscherning T, Claesson MH. Signal transduction via MHC class-I molecules in T cells. Scand J Immunol. 1994;39:117–121. doi: 10.1111/j.1365-3083.1994.tb03349.x. [DOI] [PubMed] [Google Scholar]
- 56.Yang J, Zhang X, Wang J, et al. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110:3028–3035. doi: 10.1182/blood-2007-06-094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Woodle ES, Smith DM, Bluestone JA, et al. Anti-human class I MHC antibodies induce apoptosis by a pathway that is distinct from the Fas antigen-mediated pathway. J Immunol. 1997;158:2156–2164. [PubMed] [Google Scholar]
- 58.Podar K, Tai YT, Cole CE, et al. Essential role of caveolae in interleukin-6- and insulin-like growth factor I-triggered Akt-1-mediated survival of multiple myeloma cells. J Biol Chem. 2003;278:5794–5801. doi: 10.1074/jbc.M208636200. [DOI] [PubMed] [Google Scholar]
- 59.Cherukuri A, Dykstra M, Pierce SK. Floating the raft hypothesis: lipid rafts play a role in immune cell activation. Immunity. 2001;14:657–660. doi: 10.1016/s1074-7613(01)00156-x. [DOI] [PubMed] [Google Scholar]
- 60.Unruh TL, Li H, Mutch CM, et al. Cholesterol depletion inhibits src family kinase-dependent calcium mobilization and apoptosis induced by rituximab crosslinking. Immunology. 2005;116:223–232. doi: 10.1111/j.1365-2567.2005.02213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nagy Z, Mooney N. A novel, alternative pathway of apoptosis triggered through class II major histocompatibility complex molecules. J Mol Med. 2003;81:757–765. doi: 10.1007/s00109-003-0489-9. [DOI] [PubMed] [Google Scholar]
- 62.Hardin J, MacLeod S, Grigorieva I, et al. Interleukin-6 prevents dexamethasone-induced myeloma cell death. Blood. 1994;84:3063–3070. [PubMed] [Google Scholar]