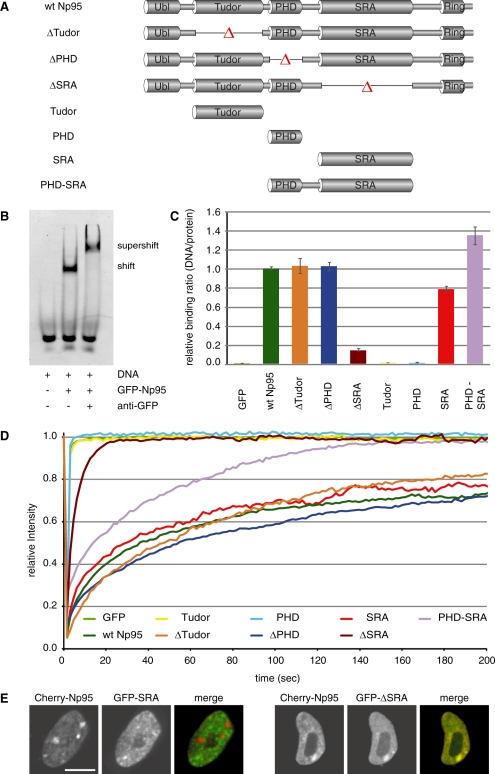
Figure 2.
In vitro DNA binding and in vivo mobility of Np95 domains. (A) Schematic representation of the analyzed GFP–Np95 fusion constructs. (B) Electrophoretic mobility shift and supershift assay. GFP–Np95 binding to hemimethylated DNA substrates is shown by the shifted GFP–Np95:DNA complex. The addition of a GFP–antibody supershifted the GFP–Np95:DNA complex (supershift assay with unmethylated DNA substrates in direct competition with hemimethylated DNA substrates is shown in Supplementary Figure S6). (C) In vitro DNA-binding properties of Np95 constructs. Binding assays were performed using fluorescently labeled double stranded oligonucleotide probes containing one central hemimethylated CpG site. Shown are fluorescence intensity ratios of bound probe/bound GFP fusion. Values represent means and SD of three to six independent experiments. GFP was used as control. Further control experiments with either one or three central CpG sites and alternating fluorescent labels are shown in Supplementary Figure S3. (D) Kinetics of Np95 constructs in living np95–/– ESCs determined by half nucleus FRAP analysis. GFP is shown as reference. Curves represent mean values from 6 to 15 nuclei. SEM (0.001–0.005) is not shown for clarity of illustration. (E) Confocal mid-sections of living np95–/– ESCs transiently expressing the indicated Np95 fusion constructs (left and mid-panels). Merged images are displayed on the right. Bar, 5 µm.