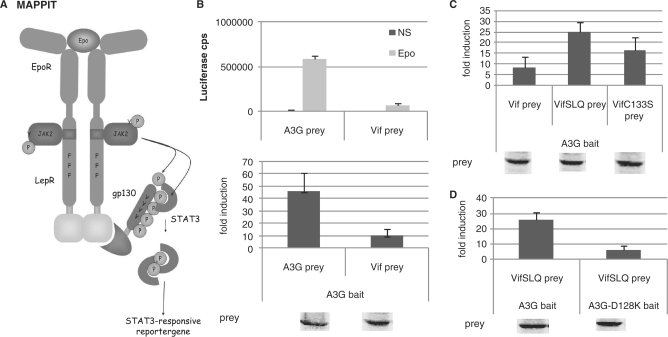
Figure 3.
(A) The MAPPIT technique is based on signal transduction of the leptin receptor. The extracellular and transmembrane part of the Epo receptor is fused to the intracellular part of the leptin receptor. The membrane-distal tyrosine at position Y1138 in the cytoplasmic tail of the receptor is replaced by phenylalanine to prevent STAT3 recruitment. The two membrane-proximal tyrosines Y985 and Y1077 are mutated to overcome negative regulation and maximize the intensity of the signal. The bait protein is attached to this signaling-deficient leptin receptor. The prey protein is fused to several STAT3 recruitment sites of the gp130 chain. Interaction of bait and prey proteins results in STAT3 recruitment and activation. A STAT3-responsive luciferase reporter gene allows detection of the interaction. (B–D) Hek293T cells were transiently co-transfected with plasmids encoding the chimeric Apobec3G bait construct (A3G bait) and the prey constructs encoding for proteins Vif, VifSLQ144-146AAA (VifSLQprey), VifC133S, A3G or A3G-D128K fused to the gp130 chain, combined with the pXP2d2-rPAP1-luci reporter. The transfected cells were either stimulated for 24 h with Epo or were left untreated (NS, not stimulated). Luciferase measurements were performed in triplicate. Data are presented as either absolute luciferase counts per second (cps) or fold induction (Epo stimulated/NS) of luciferase activity. Western blot control of the different prey constructs in the experiment is shown.