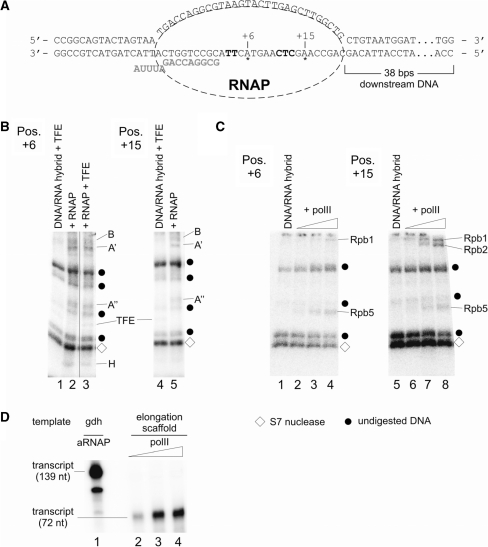
Figure 8.
Mapping of archaeal RNAP and eukaryotic polII subunits cross-linked to an elongation scaffold. The templates for this assay were assembled essentially as described (29) but contained an azidophenacylated phosphorothioate substitution at positions +6 or +15, respectively, with adjacent internal radiolabel. (A) Sequence of the template in the final assembly. RNA is marked in bold gray letters, position of the cross-linker is indicated with asterisks, and internal radiolabels are highlighted in bold letters. (B) Cross-linking of archaeal RNAP subunits to positions +6 (left) and +15 (right). ECs were formed as described in ‘Materials and Methods’ section in the presence of heparin as non-specific competitor. Lanes 1, 3 and 4 contained TFE, which cross-linked non-specifically to both templates as described (6). Radiolabeled RNAP subunits and TFE were identified by their relative electrophoretic mobility and are indicated at the right-hand side of the gels. Note that subunit H cross-links only to position +6 and not to position +15. (C) Cross-linking of polII subunits to positions +6 (left) and +15 (right) on an elongation scaffold. Control lanes 1 and 5 contained the template without polII. Triangles above lanes 2–4 and 6–8 indicate an increase of polII from 36 to 110 nM polII in the reaction. As described in (B), po lII subunits were identified by their mobility by 4–19 % SDS-PAGE. As in (B), diamonds mark auto-radiolabeled S7 nuclease, while dots specify undigested DNA. (D) Eukaryotic polII is active on elongation scaffolds. Lane 1 shows RNA products synthesized in a standard in vitro transcription assay containing 46 nM archaeal nat RNAP and Pyrococcus gdh promoter DNA. Reactions analyzed in lanes 2–4 contained increasing amounts (36–110 nM) of polII and the elongation scaffold shown in (A) as template (for details see ‘Materials and Methods’ section).