Figure 7.
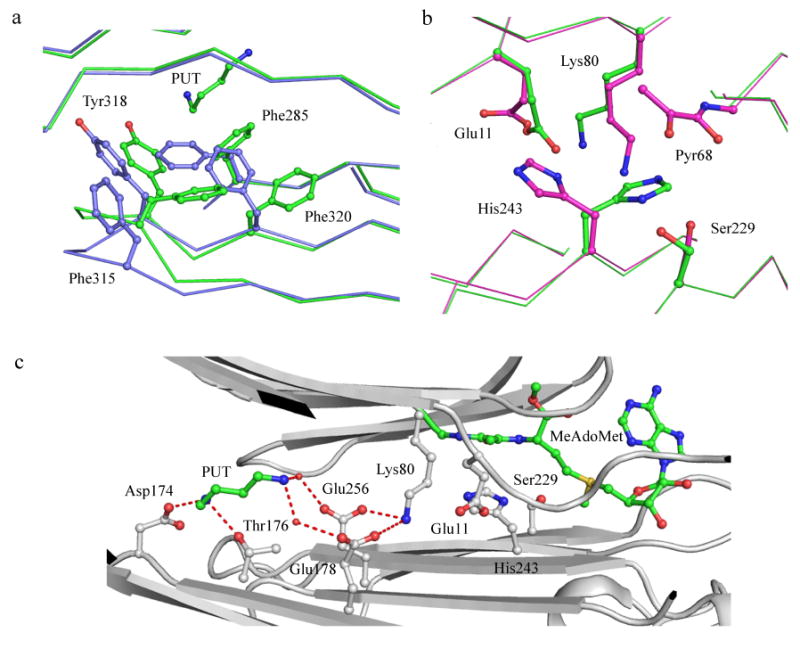
(a) Conformational changes upon putrescine binding in hAdoMetDC (PDB codes 1I7B and 3EP9). Protein with putrescine is colored green and without putrescine is colored light blue. Oxygen atoms are colored red and nitrogen atoms are colored dark blue). Putrescine and aromatic residues undergoing conformational and positional change are shown in ball and stick. (b) Alternate conformation of catalytic residues in the E256Q mutant (PDB codes 3EP4 and 3EPB). The carbon atoms of residues in active conformation are colored green and those of the inactive conformation are colored cyan. (c) Hydrogen bonding network between the putrescine binding site and the active site. All interacting residues are shown in ball and stick. Putrescine and MeAdoMet carbon atoms are colored green. Water molecules are shown as spheres and hydrogen bonds are shown as dashed lines.
