Figure 5.
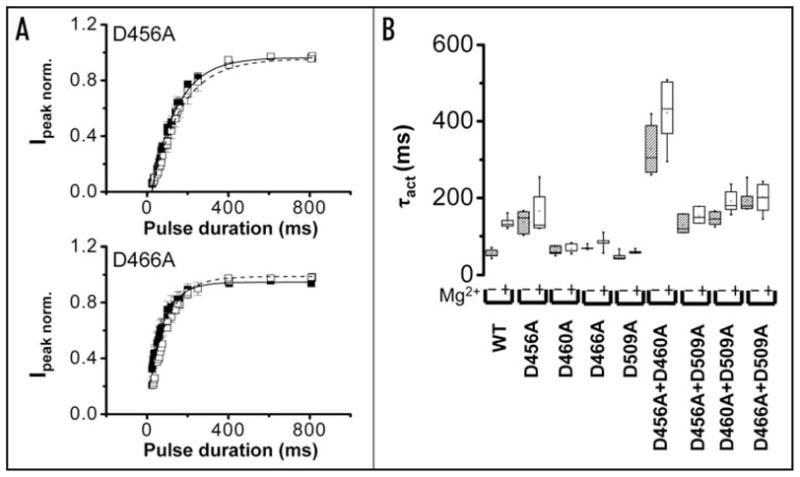
Mutating acidic residues in S2 or S3 reduces Mg2+ sensitivity of activation kinetics. (A) Using the envelope of tails procedure described in Figure 1B, normalized peak tail current amplitudes were obtained for D456A (upper) and D466A (lower) channels in the presence (□) or absence (■) of 10 mM Mg2+ and plotted versus pulse duration. Each data set was fitted with a single exponential component to obtain values for τact in the presence (dashed curve) or absence (solid curve) of Mg2+. Data are shown as mean ± SEM, n = 5. The bath solution contained 96 mM NaCl, 2 mM KCl, 0.5 mM CaCl2, 5 mM HEPES, pH 7.5. (B) Box plots of τact values obtained for wild-type and mutant channels in the absence or presence of 10 mM Mg2+ are shown. The voltage was stepped to +40 mV (+60 mV for D460A, D509A, +70 mV for double mutants) followed by repolarization to −70 mV. Values of τact for HERG wild-type and mutant channels are provided in Table 2.
