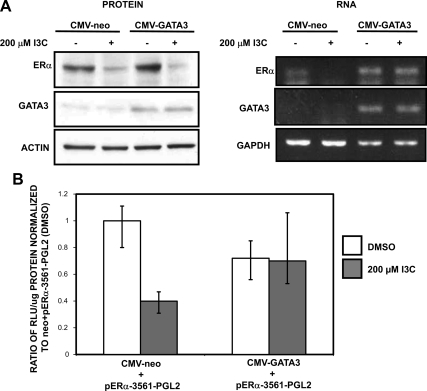
Figure 8.
The I3C inhibition of ERα transcripts levels requires the down-regulation of GATA3 gene expression. (A) MCF-7 cells were transfected with CMV-GATA3 or CMV-neo vector control and treated with or without 200 μM I3C for 48 h. Total Cell lysates were electrophoretically fractionated and analyzed by Western blots for the levels of GATA3, ERα, and actin (loading control) protein (left). Total RNA was collected from MCF-7 cells treated with or without 200 μM I3C for 24 h and RTPCR was used to detect GATA3 and ERα transcripts (right). GAPDH was used as total RNA loading control. PCR products were visualized on a 1% agarose gel stained with ethidium bromide. This result was repeated four times, and representative blots and gels are shown. (B) MCF-7 cells were cotransfected with the I3C-responsive −3561 base pairs fragment of the ERα promoter linked to a luciferase reporter plasmid along with either CMV-GATA3 or CMV-neo (vector control). At 24 h posttransfection, cells were treated with or without 200 μM I3C for 24 h, and the relative luciferase activity was evaluated in lysed cells using the Luciferase assay kit (Promega). The reporter plasmid levels are normalized to the −3561 ERα promoter fragment treated with the DMSO vehicle control. Two additional controls (data not shown) included CMV-luciferase to validate transfection efficiency (positive control) and pgl2 to measure background fluorescence (negative control). Bar graphs indicate relative luciferase activity normalized to the protein input. Error bars were derived from the results of three independent experiments.