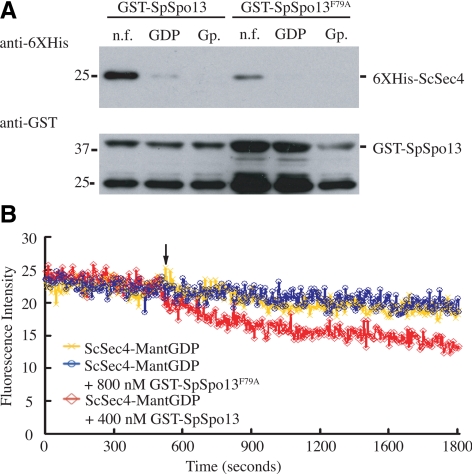
Figure 5.
Mutation of conserved residue in SpSpo13 leads to the loss of GEF activity in vitro and a FSM assembly defect in vivo. (A) GST-SpSpo13 displays reduced binding to ScSec4. GST-SpSpo13 or GST-SpSpo13F79A immobilized on glutathione-Sepharose beads was mixed with the nucleotide-free (n.f.), GDP-bound, or GppNHp-bound (Gp.) forms of 6XHis-ScSec4. The mixtures were centrifuged, and the resulting pellets analyzed by Western blot. Top, blot probed with anti-6XHis antibodies. Bottom, same samples probed with anti-GST antibodies. (B) GDP release assay. 6XHis-ScSec4 preloaded with the fluorescent GDP analogue mant-GDP was incubated in buffer containing GppNHp. At the time indicated by the arrow, buffer (yellow line), 400 nm GST-SpSpo13 (red line), or 800 nM GST-SpSpo13F79A (blue line) was added to the reaction mixture. Release of GDP was monitored by a loss of fluorescent signal from the ScSec4-mant-GDP FRET interaction.