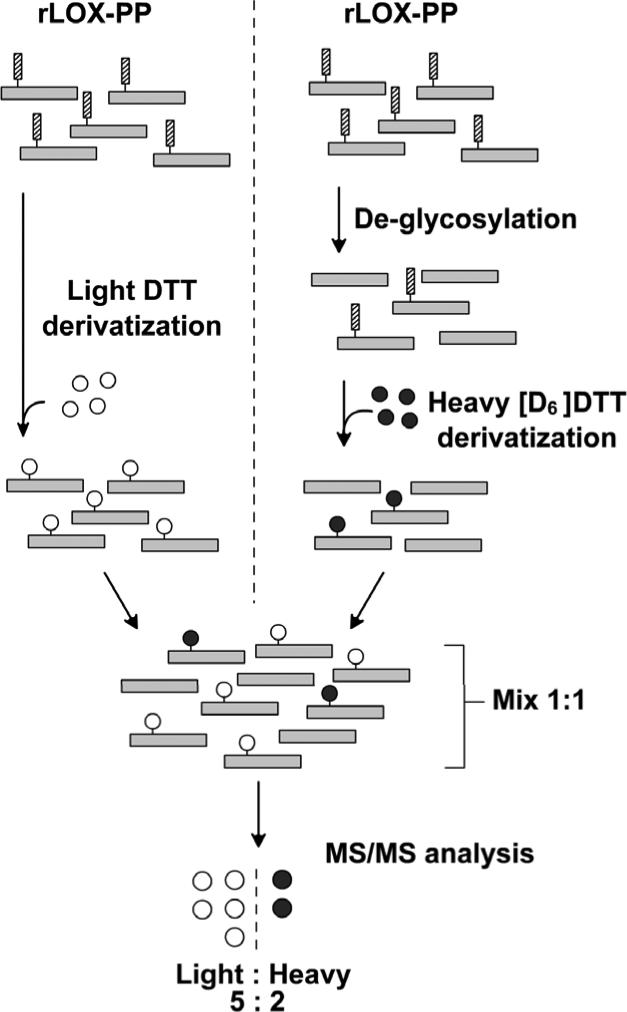
Figure 5.
Experimental design for quantitative MS/MS analysis to identify sites of O-glycosylations on LOX-PP. Separate aliquots from the same preparation of rLOX-PP were either deglycosylated or not subjected to deglycosylation. Samples were then subjected to proteolysis with chymotrypsin and Asp-N. Non-deglycosylated samples were then derivatized with DTT (open circles) whiled deglycosylated samples were derivatized with heavy [D6]DTT (filled circles). Equal amounts of non-deglycosylated and deglycosylated samples were mixed together and then subjected to LC/MS/MS. Three separate rLOX-PP preparations were subjected to this analysis. Software was instructed to recognize the same peptide fragment containing either the heavy or light DTT modification. Any Ser/Thr residue reactive with DTT but resistant to deglycosylation due to, for example, phosphorylation, would have a ratio of 1:1 of heavy and light DTT; whereas glycosylated residues were declared if the ratio of light to heavy exceeded 1.5. Data from this analysis are presented in Table 1 and summarized in Figure 6.