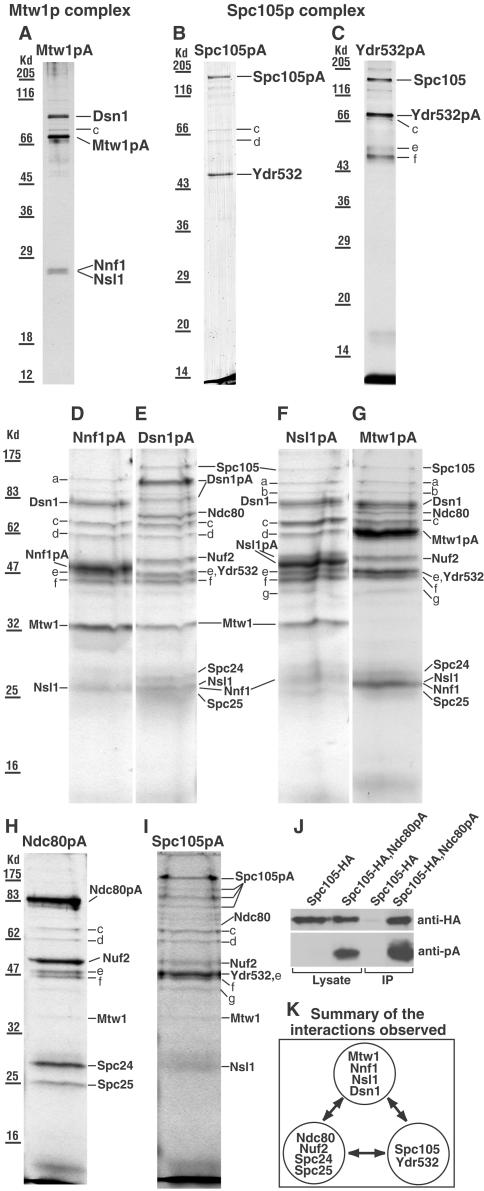
Figure 1.
(A–I) SDS gels of the complexes isolated from strains (names in brackets) containing the indicated protein A (pA)-tagged proteins. (A and G) Mtw1p (MSY5), (B and I) Spc105p (PWY282), (C) Ydr532p (MSY48), (D) Nnf1p (VNY34), (E) Dsn1p (VNY38), (F) Nsl1p (VNY32), and (H) Ndc80p (PWY350). Gels A–C were from the first fraction off the columns and gels D–I from the second fraction, which usually contains more material but also more contaminants. All the gels were Coomassie-stained except A and C, which were silver-stained. In gels D–I, the sample wells were loaded as fully as possible, which caused some distortion in the band shape. In E, the lower band labeled Dsn1pA was a proteolytic fragment since it contained peptides from both Dsn1p and protein A. In F, no further proteins were identified in the Nnf1p region of this gel. Bands a-g were identified as Rpn1p (a), Sen3p (b, only identified in gels F and G), Ssa1p, Ssa2p, or both (c), Hsp60p (d), Tef2p (e), Ydj1p (f), and Scj1p (g). Rpn1p and Sen3p are proteosome components, Ssa1p, Ssa2p, Hsp60p, Ydj1p, and Scj1p are heat shock factors, and Tef2p is elongation factor. Further information on these proteins can be found at http://www.yeastgenome.org/. It seems likely that all of these are contaminants since they have been found in isolations with noncentromeric proteins coupled to protein A (our unpublished results), and most are absent from the cleaner gels (A–C). (J) Immunoblot of immunoprecipitates (IP) obtained as described in MATERIALS AND METHODS using strains containing HA-tagged Spc105p only (VNY290), or both HA-tagged Spc105p and protein A-tagged Ndc80p (VNY296). This shows that upon enriching for Ndc80pA, trace amounts of Spc105-HA can be detected. (K) Summary of the interactions observed.