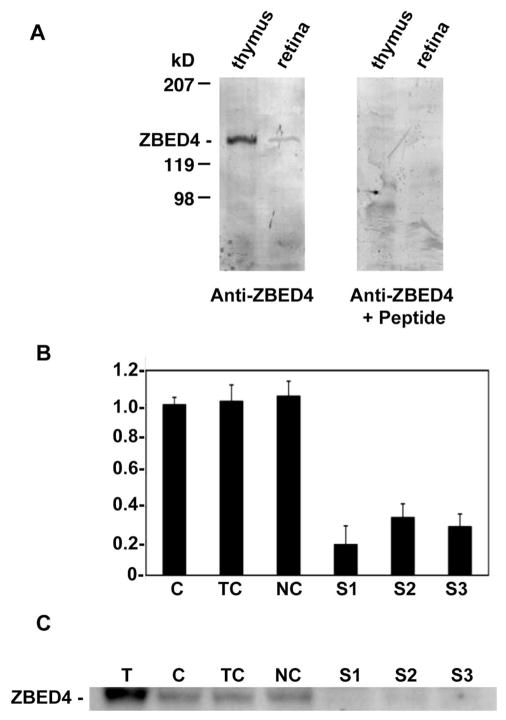
Figure 4.
Specificity of anti-ZBED4 antibodies. (A) Protein extracts from both thymus and retina were separated on a Tris/Tricine-buffered 6% acrylamide/3% cross-linking gel and transferred onto PVDF membranes. Blots were incubated with the N terminus ZBED4 antibody (left) at a 1:3000 dilution. For the competition reaction (right), the antibody was incubated overnight with a 3000 molar excess of the peptide used to generate it. The labeled band that corresponds to ZBED4, apparent molecular mass of 135 kDa, is absent in the anti-ZBED+peptide blot. Similar results were obtained with the C terminus ZBED4 antibody. (B, C) Y79 cells were transiently transfected with siRNA duplexes targeted to the 5′ or 3′ regions of ZBED4 mRNA. (B) Ninety-six hours after transfection, total RNA was extracted, reverse transcribed, and subjected to QPCR. The level of mRNA expression in all samples is relative to that in control cells (C) and shows the silencing of ZBED4 in HEK 293 cells after transiently transfecting all three siRNAs. ZBED4 antibodies barely detected or did not detect at all the expression of ZBED4 in the silenced samples. C, control cells; TC, cells with transfection reagent only; NC, negative control (totally unrelated to ZBED4 siRNA); S1, S2, S3, double-stranded ZBED4 RNA oligomers (siRNAs). (C) Protein extracts from transfected HEK 293 cell lysates were assayed for ZBED4 gene silencing by Western blot analysis.