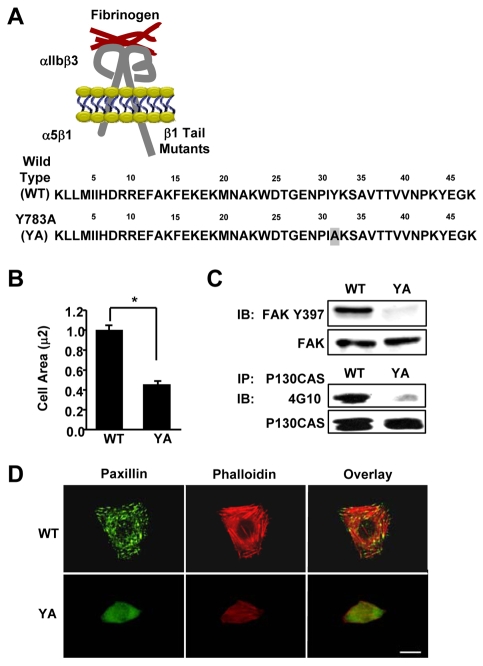
Fig. 1.
A tyrosine-to-alanine substitution in the NPIY motif of the β1A tail inhibits cell spreading, integrin signaling, and the formation of focal adhesions and stress fibers. (A) Schematic representation of the αIIlb-5/β3-1A heterodimeric chimeric integrins containing either the wild type (WT) or the Y783A (YA) mutant β1A tail with a tyrosine to alanine substitution within the membrane-proximal NPIY motif. These chimeras contain the extracellular and transmembrane domains of the αIIbβ3 fibrinogen receptor connected to the tails of the α5β1 fibronectin receptor. (B-D) CHO K1 cells stably expressing the WT or YA mutant integrin were adhered to fibrinogen for 1 hour in CCM1. (B) Cell area was measured and average cell area ± s.d. from three independent experiments is plotted, n=150 (*P<0.05). (C) Phosphorylation of FAK at Y397 was assayed by western blotting using antibodies against phosphorylated Y397 (upper panel). Blots were then stripped and reprobed for total FAK as a loading control (lower panel). p130CAS was immunoprecipitated and assayed for tyrosine phosphorylation by western blotting using monoclonal antibody 4G10. Blots were then stripped and reprobed for total immunoprecipitated p130CAS as a control. (D) Focal adhesions were visualized with antibodies to paxillin (green) and stress fibers with phalloidin (red). Scale bar: 20 μm.