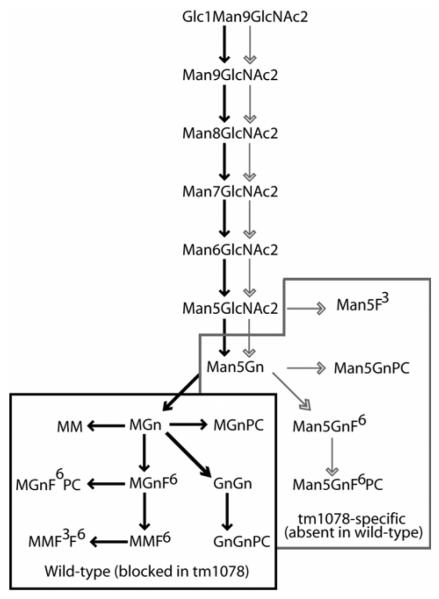
Figure 8.
Biosynthetic pathways in wild-type and aman-2 worms. Structures with Glc1Man9GlcNAc2 and Man5-9GlcNAc2 are present in both N2 and tm1078 strains and the pathways between them are shown with both solid and non-filled arrows; however, the absence of Golgi α-mannosidase II (AMAN-2) blocks the left-hand “solid arrow” route to the paucimannosidic-type mono- and difucosylated species and to the complex (in part phosphorylcholine-substituted) N-glycans found in wild-type N2 worms (shown within the black box). As judged by the accumulation of Man5Gn in aman-2 worms, the Golgi hexosaminidase is obviously unable to digest this structure in the absence of the mannosidase. On the other hand, the absence of the AMAN-2 mannosidase pushes the N-glycan biosynthesis into the right-hand “non-filled arrow” route resulting in many “non-wild type” hybrid glycans that retain the GlcNAc residue transferred by GlcNAc-TI (structures within the grey box). Furthermore, this probably allows the retention of a pool of Man5GlcNAc2 which is a substrate for the FUT-1 core α1,3-fucosyltransferase at least in vitro (the occurrence of the Man5F3 structure in vivo is putative, but is suggested by MALDI-TOF MS analysis of RP-HPLC fractions). The reduced transfer by FUT-1 to Man5GlcNAc2 (i.e., Man5) shown in vitro and the putatively reduced transfer by phosphorylcholinyltransferases to Man5Gn and Man5GnF6 would be compatible with the lower reactivity with anti-horseradish peroxidase and TEPC-15 as shown in Figure 4. The hybrid structure Man5Gn is still a substrate for the FUT-8 core α1,6-fucosyltransferase. For simplicity and due to lack of data on the exact pathways, the biosynthesis of tri- and tetrafucosylated glycans in the wild-type as well as methylated and fucosylated Hex6-8HexNAc2-3 species in the mutant is not shown. The glycan nomenclature is based on that of Schachter (3); see also Scheme I.