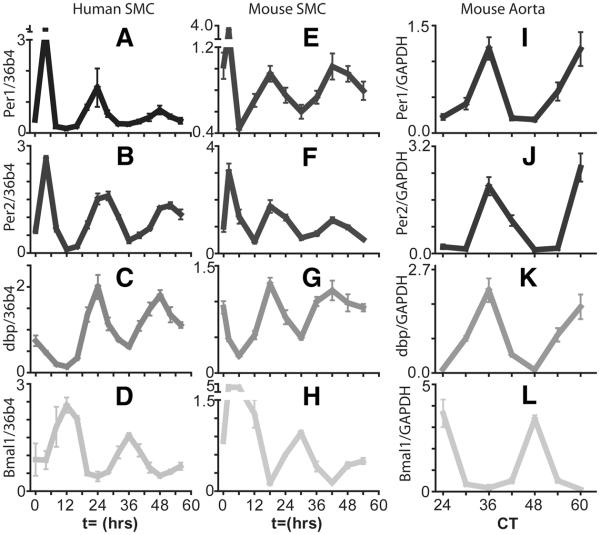
Figure 1. Accumulation of circadian clock gene transcripts in human and mouse aortic smooth muscle cells (SMC) and mouse aorta.
In the mouse aorta, clock gene mRNA transcripts Per2, Bmal1, and clock output gene dbp display circadian oscillations. Clock and Bmal1 heterodimers drive transcription of Per2 and dbp by binding to E box consensus sequences in their promoters. The peak in Per2 and dbp expression is observed around CT36 corresponding to the transition between the light and dark period. Bmal1 expression is driven by RORα acting at RRE sequences and subsequently repressed by RevErbα. The peak in Bmal1 expression is observed at CT24 corresponding to the transition from the dark phase to the light phase. Mouse aortas were harvested in constant darkness. Clock gene mRNA transcripts similarly display circadian rhythmicity in human and mouse aortic smooth muscle cells after treatment with 50% serum. The phase of the rhythms of these cells cultured in vitro is expected to be different to the in vivo rhythm due to the effect of the autonomous nervous system. Oscillation of the clock output gene dbp in smooth muscle cells is in phase with Per2 with a peak at t=24 hour post serum shock in human cells. Per2 and dbp peak slightly earlier (close to t=18 hours) in mouse smooth muscle cells. Expression of Per2, Bmal1, and dbp was monitored by qPCR. From Ref. 35 with permission.