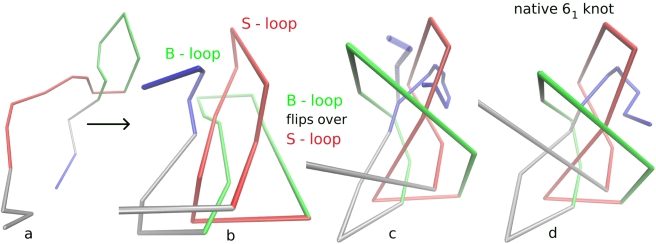
Figure 2. Snapshots taken from a folding trajectory of DehI (0→61).
The S-loop (amino acids 64 to 135) is colored red, the B-loop (amino acids 135 to 234) green and the C-terminus blue. a: B- and S-loop form in the beginning by twists of the partially unfolded protein. b: B- and S-loop align. c: the S-loop twists once again, the C-terminus threads through the S-loop (in slipknot conformation) and the B-loop flips over the S-loop. In the alternative folding scenario (0→41→61), the B-loop flips over the (twisted) S-loop before the C-terminus (indicated in light blue) threads through the S-loop (41), shortly after the C-terminus threads through the S-loop in slipknot conformation. d: Native state without slipknotted C-terminus.