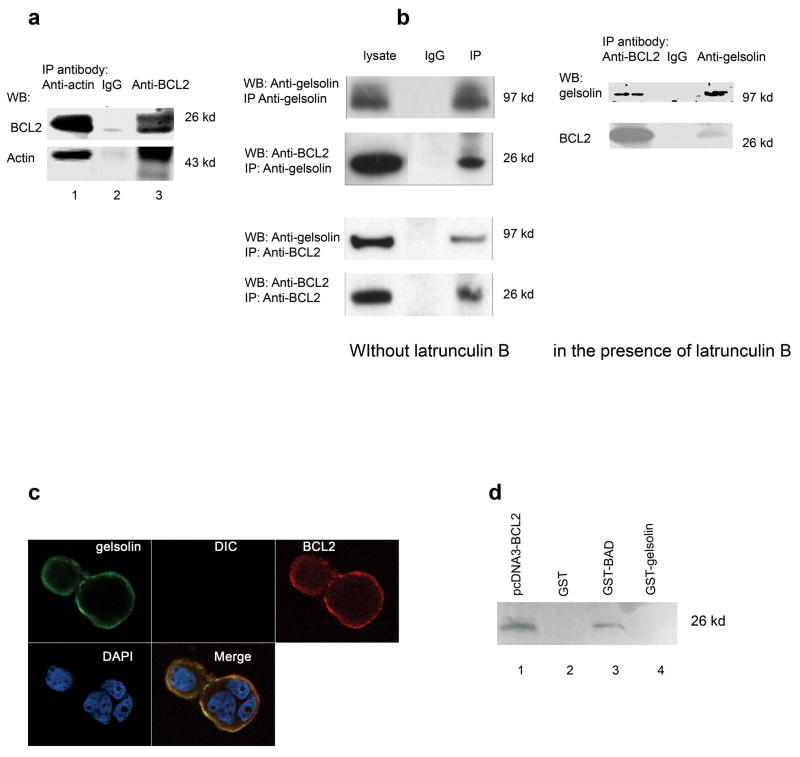
Fig. 5. BCL2 binds actin and complexes with gelsolin.
(a). Recombinant human BCL2 was mixed with actin and immunoprecipitation was carried out as described in the Materials and Methods. Both proteins were precipitated by either rabbit anti-BCL2 (human) or goat anti-Actin (human) antibody as shown in lane 1 and 3. The antibodies used in the western blot were either mouse anti-human BCL2 or rabbit anti-Actin respectively. The rabbit IgG alone control in lane 2 shows that nothing is immunoprecipitated in the absence of specific antibodies. (b). Gelsolin and BCL2 were co-immunoprecipitated from lysates of MCF-7 that overexpress BCL2 (MCF-7-BCL2 cells) with either goat anti-gelsolin or mouse anti-human BCL2 (BD Biosciences). The antibodies used in western blot were either mouse anti-gelsolin or mouse anit- human BCL2 (BD Biosciences) respectively. The experiment in the left pannel was performed in the absence of latrunculin B. The complex containing both Bcl2 and gelsolin was precipitated using either anti-gelsolin (top) or anti-BCL2 (bottom) antibody. In contrast, mouse IgG can precipitate neither gelsolin nor BCL2 (middle lanes of both top and bottom). The experiment in the right panel was performed in the presence of 0.5 μg/ml latrunculin B with similar result. (c). Confocal microscopy showed the co-localization of gelsolin with BCL2 in MCF-7-BCL2 cells. The merged image indicated both gelsolin and BCL2 were located in cell membrane and cytoplasm. These experiments were repeated twice with similar results. (d). Using the GST pull-down assays, BCL2 was found to be bound to BAD (lane 3), but did not bind directly to gelsolin in vitro (lane 4). Lane 1 showed the in vitro translated human BCL2 protein. Lane 2 showed a GST negative control. The pull-down proteins were separated by electrophoresis and visualized with Typhoon 8600 Variable Mode Imager.