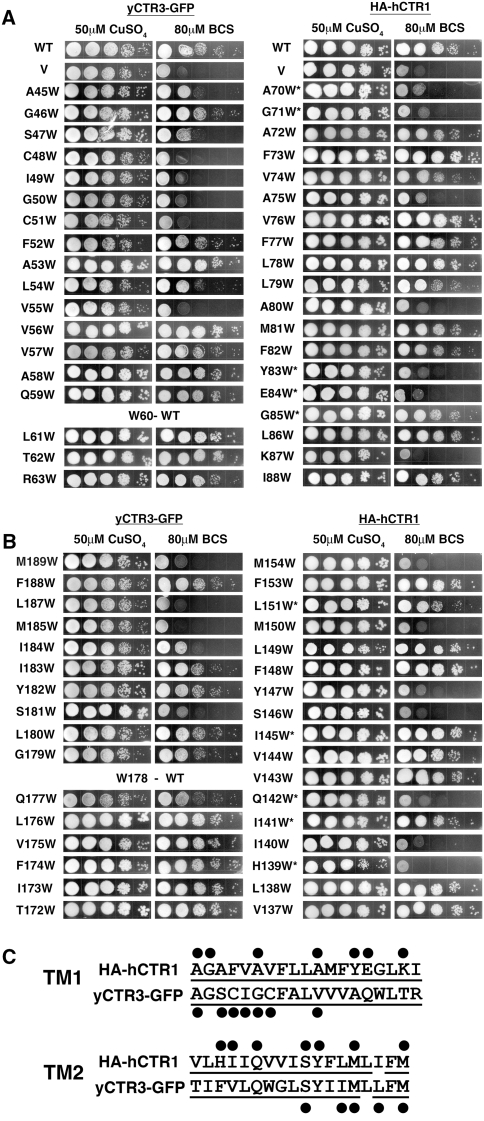
Fig. 1.
Effect of Trp mutations in TM1 and TM2 on yCTR3 and hCTR1 function. The Δctr1,3 S. cerevisiae strain, deficient in high-affinity copper uptake, was transformed with vector alone (V), wild-type (WT) yCTR3-GFP, WT HA-tagged hCTR1 (HA-hCTR1) or one of the Trp mutants. Yeast were plated in serial 1:10 dilutions on YPG, supplemented with either 50 μM copper sulfate or 80 μM of the copper chelator BCS. a, b Complementation results for TM1 (a) and TM2 (b) of yCTR3-GFP (left) and HA-hCTR1 (right). *Mutants exhibited clonal variation (see “Experimental Procedures” section). To facilitate visual comparison of spatially correlated positions, panels were mounted such that residues at the extracellular end of each helix are displayed first. c Summary of positions sensitive to Trp under the coppe-chelating conditions in the complementation assay. All residues that were assayed in this experiment are underlined and those that were sensitive to Trp are marked with a black dot