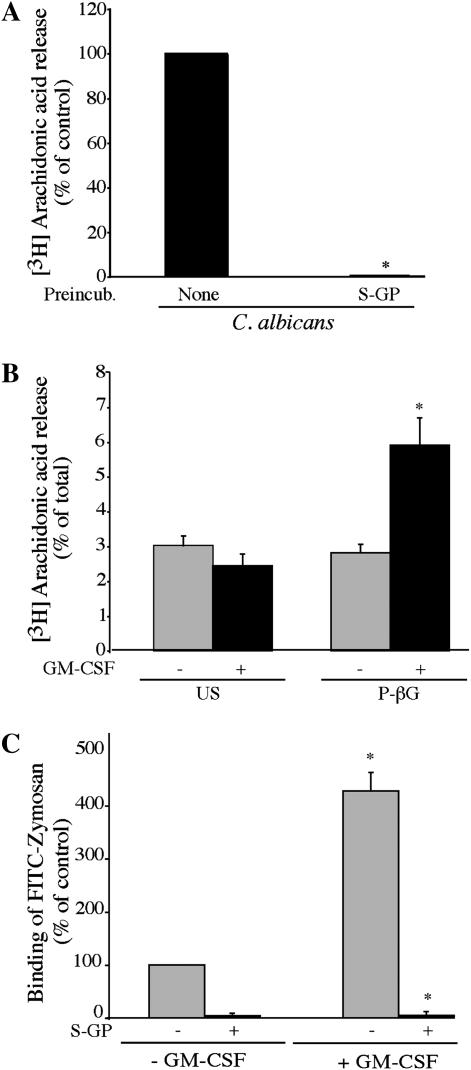
Figure 2.
A β-glucan receptor is involved in regulating AA release from alveolar macrophges. (A) [3H]AA-labeled alveolar macrophages (ICR mice) primed with GM-CSF were incubated for 30 minutes with 100 μg/ml soluble glucan-phosphate (S-GP) before infection for 60 minutes with C. albicans (moi, 20). The amount of [3H]AA released is expressed as a percentage of release from C. albicans–infected macrophages not treated with S-GP (100%). Results are the average of three experiments ± SEM. S-GP significantly (*P < 0.05) blocked [3H]AA release. (B) Unprimed or GM-CSF–primed [3H]AA-labeled alveolar macrophages were left unstimulated (US) or stimulated for 60 minutes with 100 μg/ml particulate β-glucan (P-βG). [3H]AA release from unstimulated (US) and P-βG–stimulated cells is calculated as a percentage of the total incorporated radioactivity. Data are the average ± SEM of three independent experiments. P-βG significantly (*P < 0.05) increases [3H]AA release from GM-CSF–primed cells compared with unprimed cells. (C) Unprimed or GM-CSF–primed alveolar macrophages were pre-incubated for 30 minutes with 100 μg/ml S-GP and then incubated with 25 particles/cell of FITC-zymosan at 4°C. The amount of FITC-zymosan binding is expressed as a percentage of binding to unprimed macrophages not treated with S-GP (100%). Results are the average ± SEM of three experiments. FITC-zymosan binding is significantly (*P < 0.05) higher in GM-CSF–primed macrophages relative to unprimed cells, and is significantly (*P < 0.05) blocked by S-GP.