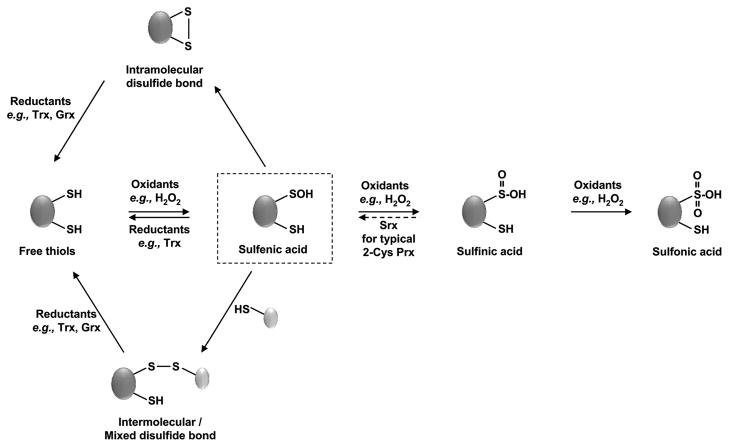
Figure 1.
Oxidative thiol modifications. Exposure of redox-sensitive cysteine residues to oxidants such as H2O2 leads to reversible sulfenic acid formation. Sulfenic acids readily react with nearby thiols of the same protein to form intramolecular disulfide bonds. They can also react with thiols of other proteins or the small tripeptide glutathione to form intermolecular or mixed disulfide bonds. Reduced cysteines can be directly oxidized to disulfide bonds by disulfide exchange reactions with oxidized glutathione (GSSG). These oxidative thiol modifications are reduced by members of the glutaredoxin (Grx) or thioredoxin (Trx) system, which draw their reducing power from cellular NADPH. In the presence of high levels of H2O2, overoxidation to sulfinic acid or sulfonic acid can occur. While sulfiredoxins (Srx) specifically reduce sulfinic acids in 2-Cys peroxiredoxins, no general sulfinic or sulfinic reductases have been identified to date.