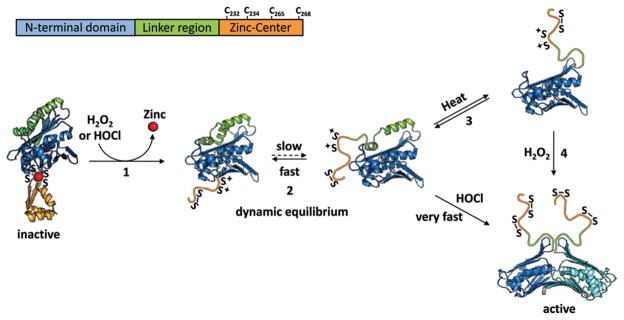
Figure 2.
Model of Hsp33’s activation. Under nonstress conditions, Hsp33 is monomeric and inactive. All four invariant cysteines are reduced and bind one zinc ion (red), forming a compactly folded zinc center (orange). (1) Exposure to oxidants causes the formation of the first disulfide bond connecting Cys265 with Cys268, which triggers zinc release and the unfolding of the zinc binding domain. (2) Unfolding of the zinc binding domain destabilizes the adjacent linker region (green), which is now in a dynamic equilibrium between a folded and partially unfolded conformation. At nonstress temperatures, the equilibrium favors the folded conformation and kinetically slow oxidants, like H2O2, cannot access the cysteines or activate Hsp33. (3) Mild denaturing conditions (e.g., elevated temperatures) shift the equilibrium and allow H2O2 to induce formation of the second disulfide bond between Cys232 and Cys234. (4) This disulfide bond apparently locks the linker region in an unfolded conformation. It causes the exposure of large hydrophobic surfaces on the N-terminal Hsp33 domain (blue), the proposed binding sites for unfolded proteins, and causes the formation of highly chaperone active Hsp33 dimers.