Figure 1.
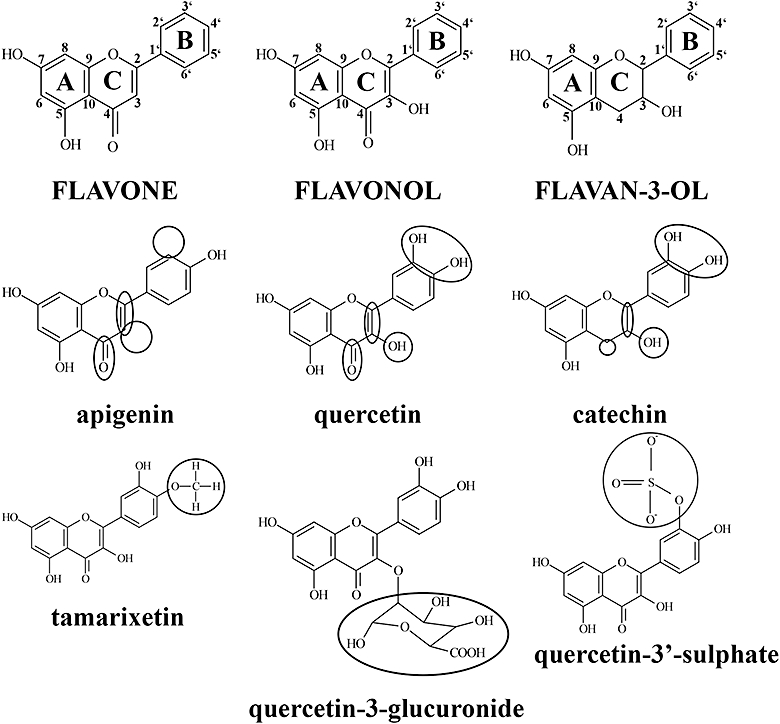
Flavonoid and metabolite structures are defined by variations in key functional groups. The flavonoid structure comprises 15 carbon atoms arranged into a 3 carbon, oxygenated heterocyclic middle ring (C ring) flanked by two aromatic rings (A and B rings). Subgroups of this family of compounds are defined by variations in functional groups integral to the flavonoid nucleus and substituted to the A, C and B rings. Flavones (apigenin) are characterized by a non-hydroxylated C ring, whereas flavonol (quercetin) C rings contain a C-3 hydroxyl group. Flavan-3-ols (catechin) are defined by a non-planar, C-3 hydroxylated C ring that is not substituted with a C-4 carbonyl group. Metabolites generated by intestinal enterocytes and liver hepatocytes are methylated (tamarixetin), sulphated (quercetin-3′-sulphate) or glucuronidated (quercetin-3-glucuronide) counterparts of parent flavonoid aglyca.
