Figure 7.
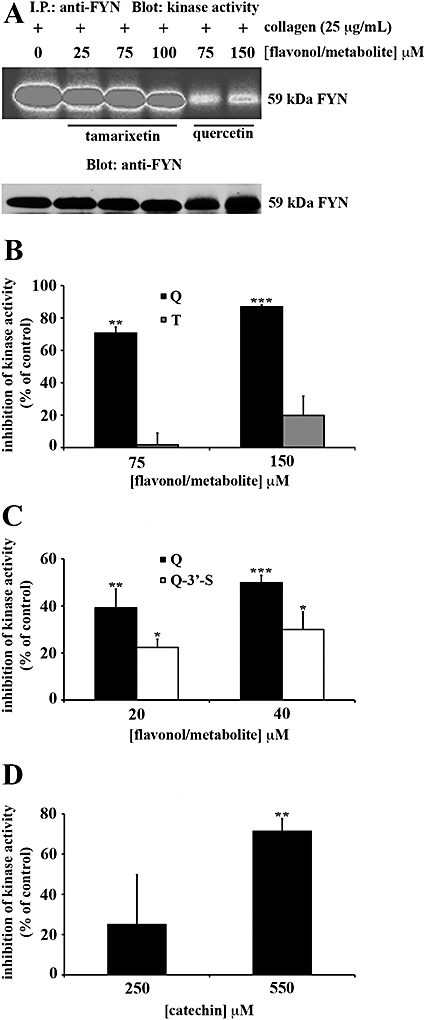
Flavonoids and metabolites block the kinase activity of Fyn in platelet lysates in a manner influenced by their pattern of hydroxylation. Washed human platelets (8 × 108 cells·mL−1) in the presence of EGTA (1 mM) were stimulated with collagen (25 µg·mL−1) for 90 s. Platelets were lysed with ice-cold 1% (v/v) NP40 and Fyn was precipitated. Platelet lysates were incubated with anti-Fyn antibody and protein G Sepharose (PGS) beads for 1 h at 4°C and Fyn immunoprecipitates were pretreated with flavonoids [quercetin (Q): A–C and catechin: D], metabolites [tamarixetin (T): A,B and quercetin-3′-sulphate (Q-3′-S): C] or solvent control [DMSO (0.2% v/v)] for 5 min. Immunoprecipitates were assayed for kinase activity as described in methods. Equivalent protein loading was verified by reprobing for Fyn. Kinase activity was expressed as a percentage of the DMSO-treated, collagen-stimulated control (100%). The bars represent the mean (n= 3) kinase activity for each treatment (±SEM). *P≤ 0.05, **P≤ 0.01 and ***P≤ 0.001 compared with the control (DMSO-treated, collagen-stimulated platelets).
