Figure 6.
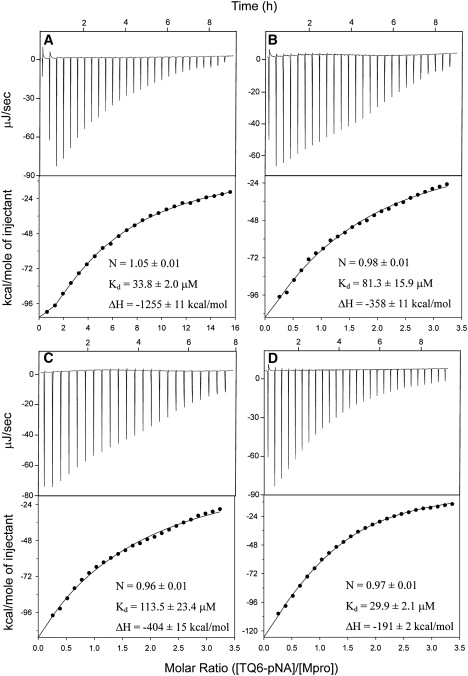
Isothermal calorimetric titration for the substrate TQ6-pNA binding to Mpro and its mutants. (A–D) Binding of TQ6-pNA to wild-type, R298A, R298L, and R298A/Q299A mutants, respectively. Wild-type protein concentration was 5.7 μM and protein concentration of the mutants was 28.6 μM. The TQ6-pNA (1 mM) was titrated into 2.7 ml of protein solution using 25–30 injections at a rate of 10 μl/injection. Values for the N, Kd, and ΔH were determined by ligand binding analysis with the Digitam program (TA instruments). The solid circles show the observed values and the lines represent fitted results.
