Figure 1.
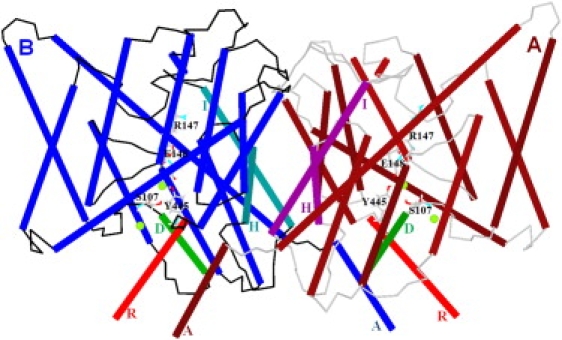
Minimized ClC-ec1 structure. View from within a membrane plane with helices in cylinder representation. Helices H and I (at the subunit interface) and helices R and D are specifically indicated for both subunits. The helices A near helices R of the other subunit are labeled. Other membrane-spanning helices are unlabeled. Polypeptide loops of subunits A and B are colored in gray and black, respectively. For clarity, crystallographic water molecules are suppressed. Four Cl− ions (intermediate-sized spheres near E148, S107, and Y 445) are shown at the central and interior binding sites of the pores. The pore lining residues S107, Y445, E148, and R147 are displayed. The E148 side-chain blocks the pore extracellularly, and side chains of S107 and Y445 constrict the pore intracellularly (30). Figs. 1–4 and 6 are generated via our MCICP code.
