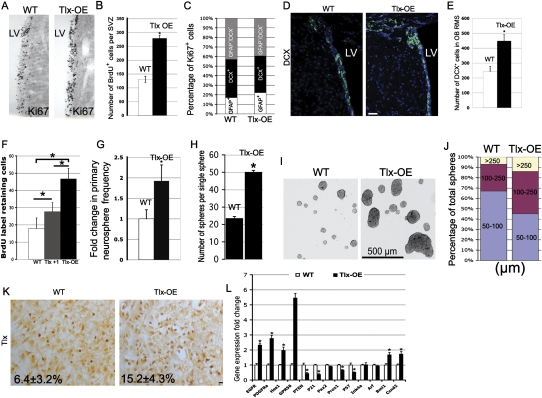
Figure 2.
Tlx overexpression leads to expansion of NSCs in vivo and in vitro. (A) Coronal brain sections of the SVZ were stained with a Ki67 antibody. Note that there are more Ki67-positive cells in the SVZ of Tlx-OE mice as compared with wild-type (WT) mice. Tlx-OE mice in this and all other figures refer to the Tlx +2 copy line. (B) Mice were sacrificed 2 h after BrdU injection, and 50-μm coronal sections were chosen for BrdU staining. BrdU-positive cells were counted and the results are shown as number of BrdU-positive cells per SVZ per section (n = 5, P < 0.01, mean ± standard deviation [SD]). (C) Confocal optical sections of coronal sections of the mouse SVZ immunostained for GFAP together with Ki67 or DCX and Ki67. Numbers of double-positive cells were counted and are presented as percentage of total Ki67-positive cells (five sections were used from each mouse, n = 3). (D) Confocal optical sections of coronal sections of the mouse SVZ immunostained for DCX. Note that more DCX-positive cells were found in the SVZ of Tlx-OE mice. (E) DCX-positive cells were counted in the RMS of the OB. Note a significant increase of DCX-positive cells in Tlx-OE mice (n = 4, P < 0.01, mean ± standard deviation [SD]). (F) Number of BrdU LRCs in the adult SVZ. Mice received BrdU continuously in the drinking water for 1 wk, followed by a 3-wk survival period. Fifty-micron coronal sections were stained with BrdU, and BrdU-positive cells were counted as described above (n = 4, P < 0.05, mean ± standard deviation [SD]). (G) Fold change of primary neurosphere frequency. Neurosphere cultures were prepared as described in the Materials and Methods, and the number of primary neurospheres was determined (n = 3, P < 0.05, mean ± standard deviation [SD]). (H) Indvidual primary neurospheres were dissociated and plated into neurosphere culture medium (one sphere per well), and the number of secondary neurospheres were counted and are presented as number of spheres per single sphere (n = 3, P < 0.05, mean ± standard deviation [SD]). (I) The size of primary neurospheres derived from the SVZ of Tlx-OE mice is bigger than in wild-type (WT) mice. (J) Percentage of neurospheres with different sizes among the total number of neurospheres. Neurospheres were categorized according to their diameter, and the percentage of different categories (50–100 μm, 100–250 μm, and >250 μm) among the total populations was quantified (at least 200 neurospheres were measured for each mouse, n = 3). (K) Tlx antibody staining of neurospheres. Note that more Tlx-expressing cells are found in neuropheres derived from Tlx-OE mice (n = 3, P < 0.05, mean ± standard deviation [SD]). (L) Quantitative PCR analysis of EGFR, PDGFRα, HES1, GPR56, PTEN, p21 (Waf1), Pax2, Prox1, P57, Ink4a, Arf, Bmi1, and Ccnd2 mRNA expression in microdissected SVZ cells of the adult SVZ of wild-type and Tlx-OE mice (n = 5, P < 0.05, mean ± standard deviation [SD]).