Table 2.
| entry | substrate | endo product | exo product | endo:exob | yield (%)c |
|---|---|---|---|---|---|
| 1 |
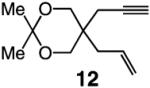
|

|
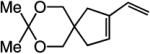
|
>98:<2 | 81 |
| 2 |
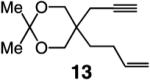
|
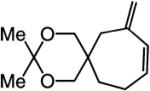
|
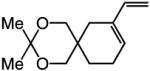
|
75:25 | 75d |
| 3 |
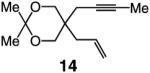
|
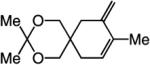
|
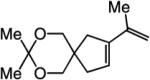
|
66:33 | 72d |
| 4 |
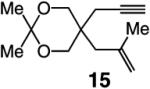
|
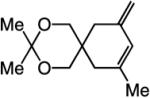
|
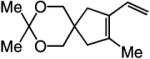
|
>98:<2 | 81 |
Reactions were performed under N2 atmosphere with 5 mol % 5 for 30 min in C6H6 at 22 °C.
Ratios were determined by analysis of 400 MHz 1H NMR spectra of unpurified mixtures prior to purification.
Yield of isolated products after purification.
Combined yields of the product mixture.
