Figure 7.
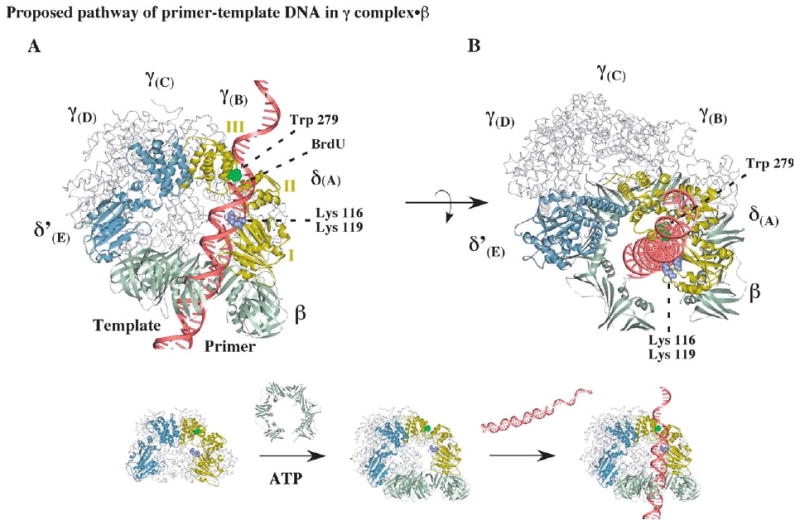
Possible positioning of primed DNA within the clamp loader-open clamp complex during clamp assembly. (A) A model of the open β clamp docked onto γ complex, illustrating a possible path for primer-template DNA with the duplex portion passing through the β ring and the single-stranded portion exiting the complex near the C-terminal domain III of δ. The structure of δ is shown with the DNA-cross-linking residue, tryptophan 279, colored green and lysine 116 and lysine 119 colored purple (the alphabet nomenclature for γ complex subunits corresponds to that used for the S. cerevisiae RFC crystal structure).11 (B) Another view of the complex, illustrating possible stacking of tryptophan 279 against the primer-template junction.
