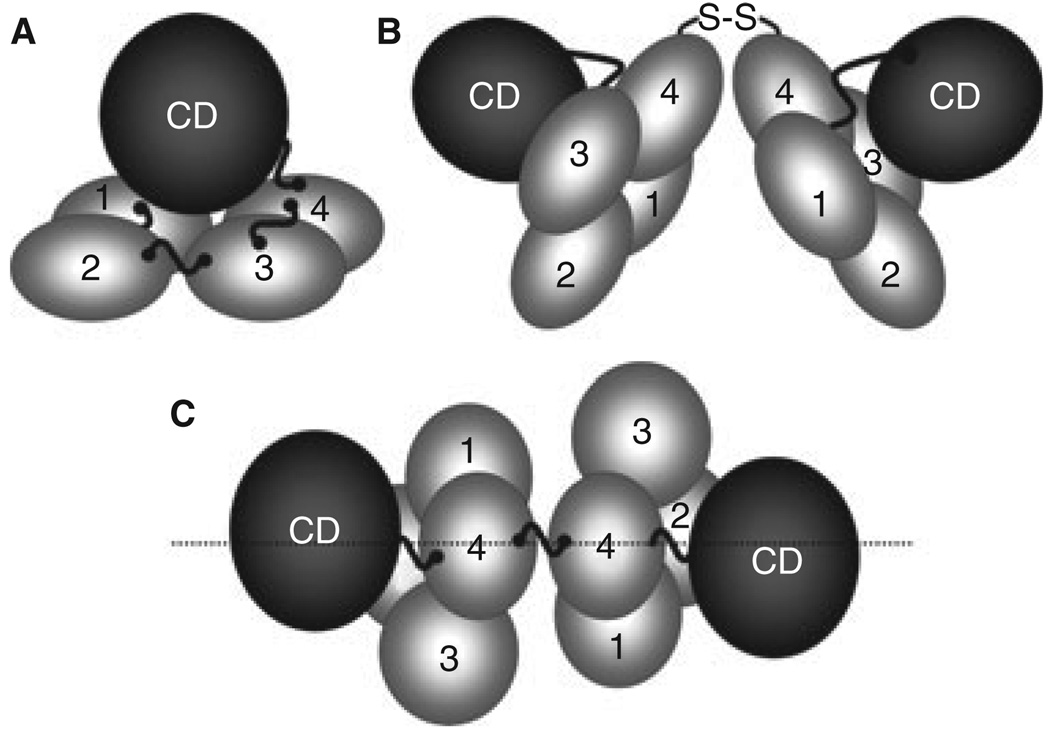
Fig. 2.
Factor XI Structure. (A) A factor (F) XI subunit contains four apple domains (1–4) that form a planar structure on which the catalytic domain (CD) rests. High molecular weight kininogen likely binds to the underside of the apple domain disc. Cleavage at Arg369-Ile370 between A4 and the CD generates FXIa. (B) FXI is a dimer of two identical subunits, with the A4 domains forming the interface. (C) In this view of the dimer from above the A4 domains, the positions of the two A3 domains on opposite sides of the longitudinal plane of the molecule can be seen.