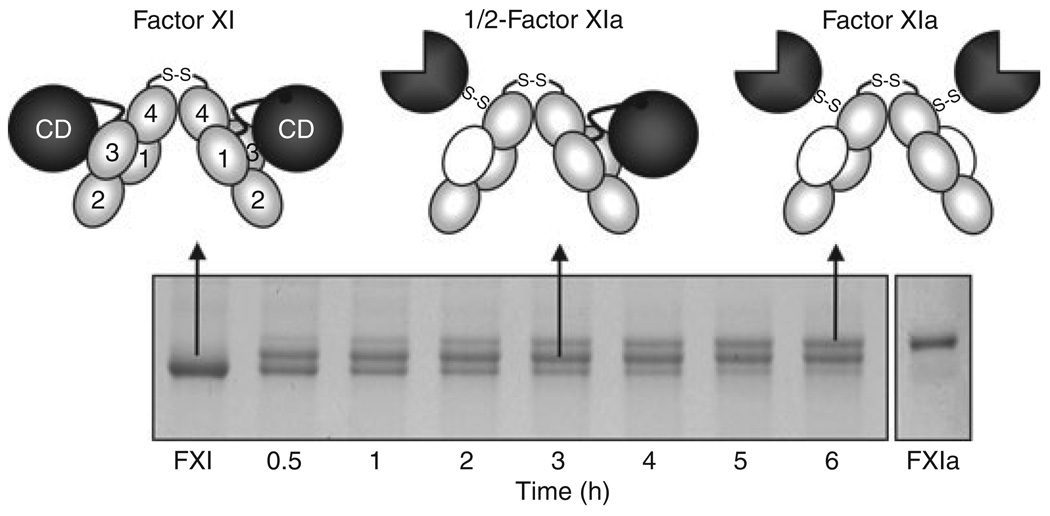
Fig. 3.
Factor XI Activation. Each factor (F) XI subunit is activated by cleavage between Arg369 and Ile370. FXI migrates slightly faster than FXIa on SDS–PAGE. Activation of FXI by α-thrombin (shown) or FXIIa proceeds through an intermediate in which only one dimer subunit is cleaved (1/2-FXIa). Subsequent conversion of 1/2-FXIa to fully activated FXIa appears to be a slower process. In the schematic diagrams at the top of the figure, gray ovals indicate apple (A) domains, black circles unactivated catalytic domains, and three-quarter circles activate catalytic domains. Note that with activation, an exosite for factor IX binding is exposed on the A3 domain (indicated by white ovals).