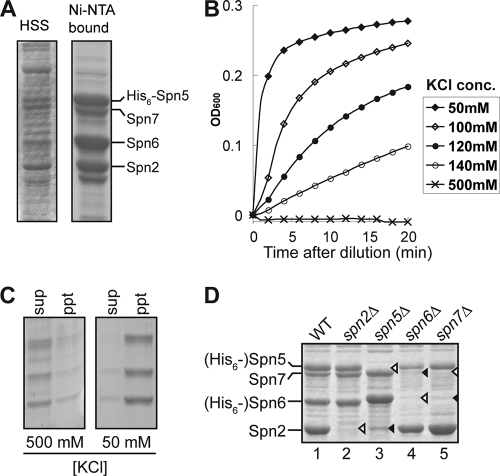
FIG. 7.
Formation of complexes by sporulation septins. (A) His6-Spn5, Spn2, Spn6, and Spn7 were coexpressed in bacteria and extracted as described in Materials and Methods. The high-speed supernatant (HSS) and the complex purified by Ni-nitrilotriacetic acid (Ni-NTA) binding were analyzed by SDS-PAGE and Coomassie staining. (B and C) Polymerization of the purified septin complexes from panel A at lowered KCl concentrations was monitored by measuring turbidity (A600) as a function of time (B) and by centrifuging samples from the 10-min time point at 100,000 × g for 1 h and analyzing the supernatants (sup) and precipitates (ppt) as in panel A (C). (D) Analysis of septin complexes lacking a subunit. His6-Spn5 was coexpressed with Spn2, Spn6, and Spn7 (lane 1), with Spn6 and Spn7 (lane 2), with Spn2 and Spn7 (lane 4), or with Spn2 and Spn6 (lane 5), and His6-Spn6 was coexpressed with Spn2 and Spn7 (lane 3). The complexes were analyzed as in panel A. Open arrowheads, septins not expressed in particular samples; closed arrowheads, septins that were present but did not copurify with the His6-tagged subunit.