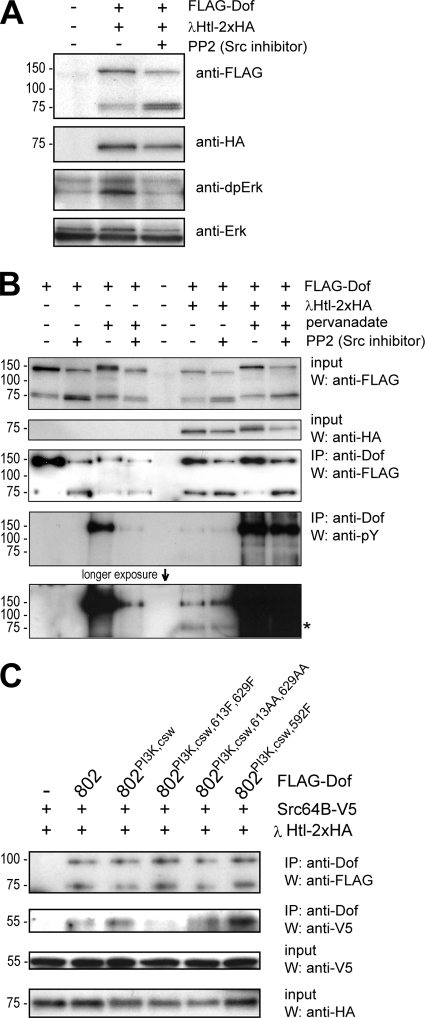
FIG. 6.
Phosphotyrosine-dependent interaction of Dof with Src64B. (A) The relevance of Src kinase activity in FGFR-dependent MAPK activation was tested in S2 cells. FLAG epitope-tagged Dof was expressed together with an activated form of HA-tagged Htl (λHtl-2xHA) in the presence or absence of the Src kinase inhibitor PP2. MAPK activation was detected by Western blotting using an antibody against the diphosphorylated form of Erk. (B) The involvement of Src kinases in Dof phosphorylation was monitored in S2 cells. FLAG-Dof was expressed alone or together with λHtl-2xHA in the presence or absence of tyrosine phosphatase and Src kinase inhibitors pervanadate and PP2. The phosphorylation state of the Dof protein was determined by a phosphotyrosine antibody after immunoprecipitation (IP) from whole-cell lysates using a polyclonal Dof antibody. The asterisk marks the tyrosine-phosphorylated λHtl-2xHA coprecipitating with FLAG-Dof. Note that the apparent molecular weights for the Dof constructs appear not to be identical, although the constructs are the same in all lanes. We assume this is due to phosphorylation, as the migration behavior in the gel correlates with the phosphorylation state. W, West- ern blot analysis. (C) The ability of Src64B to interact with Dof and the requirement of phosphorylated tyrosines and novel tyrosine motifs in Dof for such an interaction were tested in S2 cells. Dof mutants 802, 802[PI3K,csw], 802[PI3K,csw,613F,629F], 802[PI3K,csw,613AA,629AA], and 802[PI3K,csw,597F] were expressed together with λHtl-2xHA and a V5 epitope-tagged form of Src64B (Src64B-V5). Dof protein complexes were immunoprecipitated from whole-cell lysates using an antibody against Dof and the precipitated proteins were detected by Western blotting using antibodies directed against FLAG or V5. Expression levels of Src64B-V5 and λHtl-2xHA were controlled by Western analysis of whole-cell lysates using antibodies against the V5 and HA epitope tags.