FIG. 11.
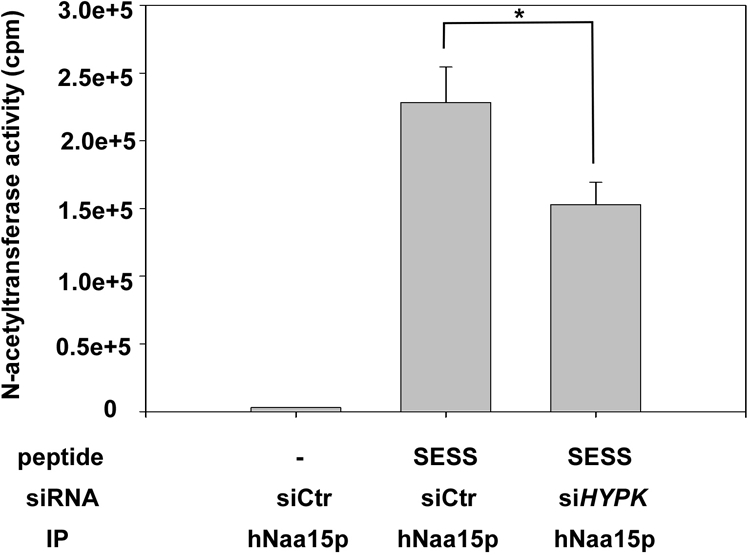
hNatA immunoprecipitated from HYPK knockdown cells displays reduced in vitro acetylation activity. HeLa cells (approximately 5 × 106 per sample) were transfected with the indicated siRNAs, and at 24 h posttransfection, ZVAD was added to prevent induction of apoptosis. After 48 h, the cells were harvested and lysed, and the lysate was subjected to immunoprecipitation (IP) using an anti-hNaa15p specific antibody. The beads containing functional hNatA complexes were analyzed for Nα-acetyltransferase activity, using [14C]acetyl-CoA and a Ser-Asp-Ser-Ser (SESS)-starting 24-mer peptide known to be acetylated by NatA in vitro. The amount of acetyl incorporation was determined by isolation of the peptides followed by scintillation counting. Verification of knockdown and the presence of equal levels of hNaa15p in the immunoprecipitates were routinely confirmed. Experiments were performed three times, and values are means ± SD. P values for independent t tests for siHYPK samples versus control are indicated with an asterisk (P < 0.05).
