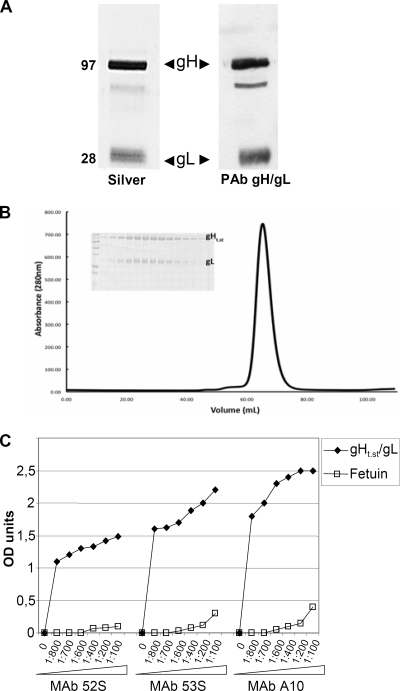
FIG. 1.
Properties of purified gHt.st/gL. (A) Aliquots of gHt.st/gL made in insect cells and purified by means of Strep-Tactin resin according to the manufacturer protocol were separated by denaturing polyacrylamide gel electrophoresis and silver stained (Silver) (left lane) or reacted by Western blotting with a PAb to gH/gL (right lane). The values to the left are molecular sizes in kilodaltons. (B) Size exclusion chromatography on a Superdex 200 column (GE Healthcare) in 10 mM Tris (pH 8.0)-150 mM NaCl. Static light scattering was carried out over the gHt.st/gL peak during elution from the Superdex 200 column to determine the molecular weight of purified gHt.st/gL. The x axis is elution volume in ml, and the y axis is milliunits of absorbance at 280 nm (mAU). Insert, pattern of proteins as seen in denaturing gel electrophoresis with Coomassie blue staining. (C) ELISA reactivity of gHt.st/gL to neutralizing MAbs 52S, 53S, and A10 to gH/gL. gHt.st/gL or fetuin, as a negative control, was immobilized on 96-well plates and allowed to react with increasing dilutions of the MAbs. Reactivity was detected by means of anti-mouse antibodies conjugated to peroxidase, followed by o-phenylenediamine and reading of the optical density (OD) at 490 nm. Each point represents the average of triplicate measurements.