Figure 1.
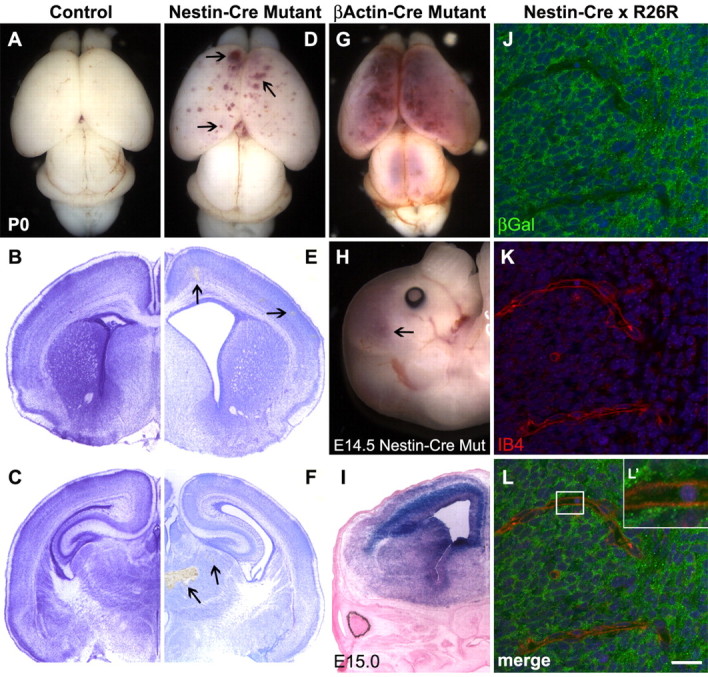
Phenotypic defects observed after conditional deletion of itgβ8 from the neuroepithelium using nestin-cre. A, Wild-type brain from P0 neonate. B, C, Nissl-stained sections of a control brain. D, P0 brain lacking itgβ8 because of conditional deletion mediated by neuroepithelial-specific nestin-cre. Note the blood produced from hemorrhages throughout the dorsal and rostral cortices (arrowheads). E, F, Nissl-stained sections of a nestin-cre mutant brain. Notice the hemorrhages (arrowheads) throughout the cortex (E) in addition to the massive hemorrhage in the thalamus (F). G, P0 mutant animal generated using the β-actin-cre line. Mutants generated with this cre line display phenotypes not obviously different from the null animal. An avas-cularized yolk sac and placenta is seen in the majority of embryos that die by E11.5, and severe hemorrhage is seen in the brain of embryos that survive to birth. H, E14.5 brain lacking itgβ8 from the neuroepithelium because of conditional deletion mediated by neuroepithelial-specific nestin-cre. Note the blood produced from hemorrhages throughout the dorsal and rostral cortices (arrowhead). I, LacZ-stained section from an E15 nestin-cre-positive animal crossed to the R26R reporter strain. Note that the neuroepithelium is almost entirely recombined. J-L′, β-galactosidase expression analysis of E15 embryo cortices (one-half day after hemorrhage was observed in nestin-cre mutants) obtained from a cross of a nestin-cre-positive animal and the R26R reporter line. Note the strong expression of β-galactosidase (green) in cells of the neuroepithelium but the lack of β-galactosidase immunolabeling of the endothelial cells labeled with isolectin B4 (red) (L′). Cell nuclei were counterstained with TO-PRO-3 (blue). βGal, β-Galactosidase; Mut, mutant; IB4, isolectin B4. Scale bar: L, 20 μm.
