Figure 2.
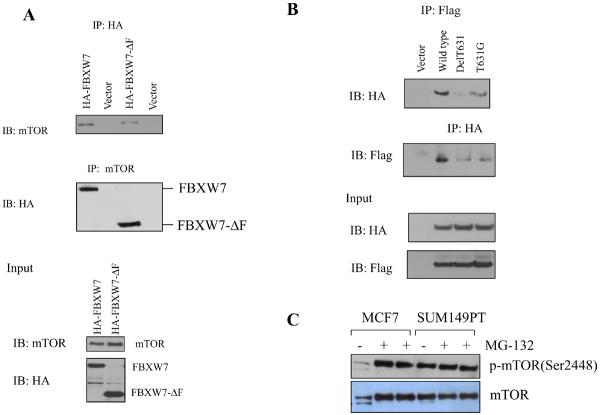
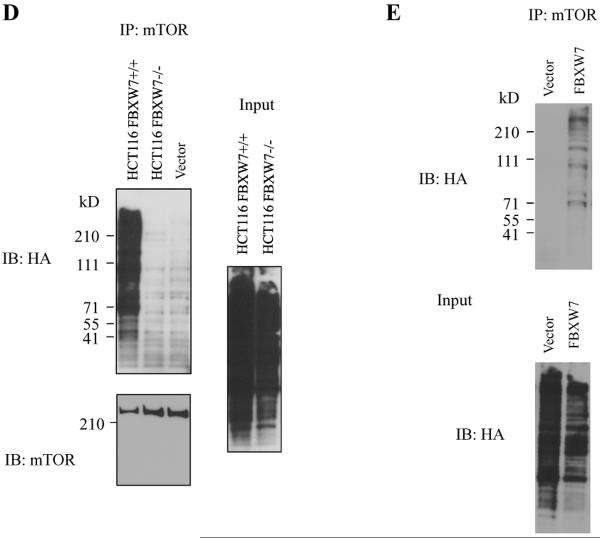
mTOR interacts with FBXW7. (A) Immunoprecipitation of HA tagged FBXW7 identifies mTOR as an interacting protein. HA-tagged FBXW7 or FBXW7-Δ were expressed in human 293T cells, followed by immunoprecipitation of the proteins with anti-HA antibodies and immunoblotting with antibodies against mTOR (top panel). The reciprocal experiment shows that the region of FBXW7 that interacts is the WD40 domain (middle panel). Lower panel shows the input levels of mTOR and FBXW7 proteins in the lysates. (B) FBXW7 binds the wild-type fragment of mTOR, but binding to the mTOR(delT631) and mTOR(T631G) mutants was dramatically reduced. (C) MCF7 breast cancer cells show increased mTOR levels upon treatment with MG-132, but this is not seen in SUM149PT cells which have no functional FBXW7 gene. (D) HCT116_WT and HCT116_FBXW7−/− cells were transfected with HA-ubiquitin. Immunoprecipitation of mTOR followed by Western blot analysis of the HA-ubiquitin showed that ubiquitination of mTOR was only seen in the HCT116_WT cells, and not in HCT116_FBXW7−/− cells. The vector lane shows HCT116_FBXW7−/− cells transfected with empty vector construct. (E) Ubiquitination of mTOR is restored by exogenous FBXW7 expression. HCT116_FBXW7−/− cells were transfected with an FBXW7-expressing construct and HA-tagged ubiquitin. Immunoprecipitation of mTOR showed increased ubiquitination compared to controls (fig. S7).