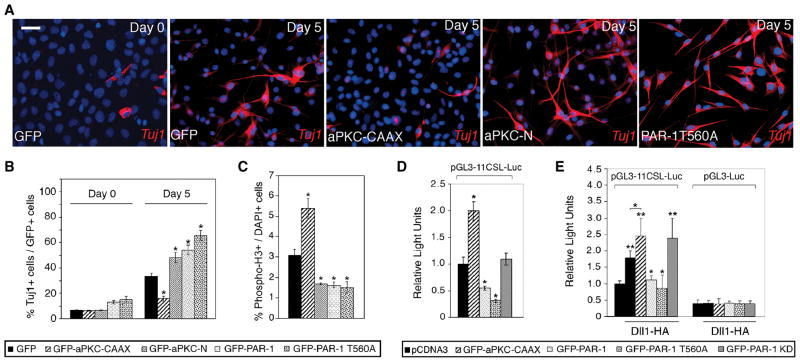
Figure 2. PAR-1 and aPKC regulate neurogenesis in mammalian neural progenitors.
C17.2 cells infected with lentiviruses expressing GFP or different aPKC and PAR-1 constructs were allowed to differentiate for five days. (A) Representative images of cells stained for TuJ1 (red) and DAPI (blue) before and after differentiation. Neuronal differentiation is revealed by bipolar morphology of cells, containing long processes, and TuJ1 staining. PAR-1, PAR-1T560A and aPKC-N stimulated, whereas aPKC-CAAX suppressed neuronal differentiation. Scale bar, 20 μm. (B) Summary of experiments shown in (A). *, p<0.01, indicates significant differences from the control GFP group. (C) Phospho-histone H3 positive cells in C17.2 cultures expressing indicated lentiviral constructs. PAR-1, PAR-1T560A and aPKC-N decreased, whereas aPKC-CAAX increased the number of mitotic cells. Data are from three independent experiments expressed as ratio of PH3+/DAPI+ cells. *, p<0.01 indicates significant differences from the control GFP group. (D) aPKC and PAR-1 regulate Notch reporter activity in neural progenitor cells. C17.2 cells were transfected with aPKC and PAR-1 constructs as indicated, and the pGL3-11CSL-Luc reporter and harvested 24 hrs later for luciferase activity measurement. Relative luciferase activity was normalized to Renilla enzyme activity. (B–E) Data are presented as the means ± s. d. of three independent experiments. *, p<0.05. (E) PAR-1 inhibits Dll1 activity in signal-sending cells. HEK293T cells were transfected with Dll1 and aPKC or PAR-1 constructs, then cocultured for 24 hrs with NIH3T3 cells expressing Notch and pGL3-11CSL-Luc reporter. pGL3-Luc is a control reporter. Each group contained triplicate samples. **, p<0.01, or *, p<0.05, indicate significant differences from the control- or the Dll1-transfected group, respectively.