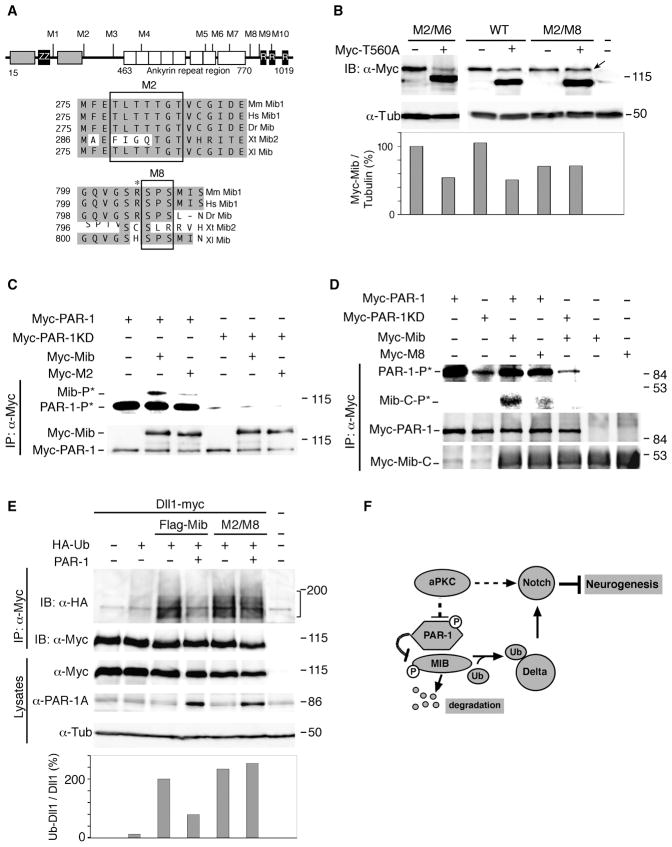
Figure 6. PAR-1 influences Dll1 ubiquitination by phosphorylating Mib.
(A) Putative PAR-1 phosphorylation sites in the Mib protein. The HERC2 domain (grey box), zinc finger regions, ZZ-type (ZZ) and ring-type (R), and the ankyrin repeat regions are shown. Alignment of the M2 and M8 Mib region that are critical for PAR-1-dependent degradation. Asterisk marks positively charged residue upstream of S804/S806. Sequence accession numbers: Mm Mib1, NM_144860; Hs Mib1, NP_065825; Dr Mib, AF537301; Xt Mib1, BC167461, Xt Mib2, NP_001123444; Xl Mib, NP_001085805. (B) Mib M2/M8 is insensitive to downregulation by PAR-1. Embryos were injected with indicated RNAs at the two-cell stage for western analysis at stage 10.5. Mib (top band) and PAR-1T560A (bottom band) are detected with anti-Myc antibodies. α-tubulin is a control for loading. (C, D) Decreased phosphorylation of Mib proteins with mutated M2 (C) and M8 (D) sites as compared to wild-type Mib. Arrow points to lack of decrease in M2/M8 levels in presence of PAR-1. (C) M2 mutations are in the context of the full-length protein. (D) Phosphorylation of Mib-M8 mutant is analyzed in the context of a carboxy-terminal fragment of Mib (amino acids 691–1031) which migrates faster than PAR-1. Carboxy-terminal fragments of the wild-type Mib or M8 mutants are compared. The details of the immune complex kinase assay are as in Fig. 3B legend. (E) M8* Mib promotes Dll1 ubiquitination in a PAR-1-independent manner. Embryos were injected with Dll1-myc (0.3 ng), HA-Ub (1 ng), PAR-1 (0.4 ng), Flag-Mib or Flag-M8* Mib (0.3 ng) RNAs as indicated. Dll1 ubiquitination was analyzed as described in Fig. 4A. Compared to Fig. 4A, image was acquired at a different exposure time, since Delta is strongly ubiquitinated by Flag-Mib. Lower panel shows ratios of ubiquitinated (HA-tagged) Dll1 to total (myc-tagged) Dll1 in immunoprecipitates. (F) A model for regulation of neurogenesis by PAR-1 and aPKC. aPKC promotes Notch signaling and negatively regulates PAR-1 activity in neural progenitors. PAR-1 phosphorylates Mib leading to its degradation, suppressed Dll1 monoubiquitination, inhibited Notch signaling and promoted neurogenesis.