As primary conductors of ionic charge across virtually every cell in our body, ion channels play a fundamental role in various aspects of mammalian physiology. The role of ion channels in physiology is particularly evident in the function of the heart and the brain which operate primarily by conducting electrical impulses. Not surprisingly, investigations of mechanisms associated with ion channel function have dominated basic science research in areas of cardiology and neurology. However, we know very little about the mechanisms involved in intracellular delivery and the localization of ion channels. A mislocalized ion channel is not only physiologically worthless, its altered localization may be detrimental to the cell and the overall survival of the organism.
In a recent issue of The Journal of Physiology, Zadeh et al. (2009) investigated the molecular mechanisms implicated in the delivery of a cardiac voltage-gated potassium channel (Kv1.5) to its final destination, the plasma membrane. They found that a particular isoform (Kif5b) of the kinesin superfamily is involved in the transport of the Kv1.5 channels to the cell surface. Prior to this study, it was well known that kinesin motor proteins are critical for the transport of ion channels across lengthy distances in axons of nerve cells (Rivera et al. 2007). However, the study of Zadeh et al. (2009) suggests that kinesins are equally involved in the transport of ion channels in cardiac cells.
Kinesins and dyneins are motor proteins which deliver a large cargo to their final cellular destination by moving along the microtubule tracks. As depicted in Fig. 1, in general, kinesins move from the centre of the cell towards the periphery (or the plus end of microtubules) and dyneins move in the reverse direction. Kinesin-1 (also known as conventional kinesin) is perhaps the most prominent member of the kinesin superfamily. Kinesin-1 is a tetrameric complex composed of two heavy chains (KHC) and two light chains (KLC). The amino terminal end of KHC interacts with the microtubule and hydrolyses ATP to generate the force for movement. Binding to cargo is mediated by either KHC or KLC (reviewed in Hirokawa & Noda, 2008).
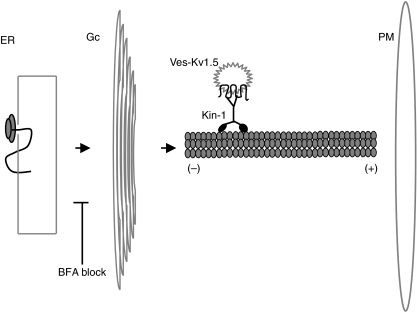
Figure 1. Synthesis and transport of Kv1.5 channels.
Schematic diagram of the secretory pathway indicating key compartments relevant to protein synthesis and transport. Integration of the nascent Kv1.5 polypeptide into the membrane of the endoplasmic reticulum (ER) takes place during translation by ribosomes associated with the ER. The maturation of Kv1.5 polypeptides continues as they exit the ER and transit the Golgi complex (Gc) by vesicle fission and fusion. According to the model proposed by Zadeh et al. (2009), the delivery of vesicles containing Kv1.5 channels (Ves-Kv1.5) from the Gc to the plasma membrane (PM) is mediated by the movement of kinesin-1 (Kin-1) toward the plus (+) end of the microtubule track. Block of ER exit by BFA (as shown) eliminates the ability of Kin-1 to deliver Ves-Kv1.5 to PM, suggesting that Kin-1 operates between the Gc and the PM.
Zadeh et al. (2009) focused their investigation on Kif5b, the only KHC subunit of kinesin-1 which is ubiquitously expressed (Hirokawa & Noda, 2008). They found that overexpression of Kif5b results in a significant increase in functional expression of Kv1.5, presumably because more channels are delivered to the plasma membrane. The delivery of Kv1.5 channels by Kif5b is expected to take place between the Golgi and the plasma membrane (as shown in Fig. 1). Therefore, manipulations that block transit to Golgi should lessen or eliminate the increase in functional expression of Kv1.5 by Kif5b. Zadeh et al. (2009) show that blocking the exit of Kv1.5 channels from the endoplasmic reticulum by brefeldin A (BFA) attenuates the increase in functional expression of Kv1.5 by Kif5b. In order to more directly test the specific role of Kif5b, in several of their experiments the authors compared the effect of overexpressing Kif5bDN, a dominant negative construct of Kif5b. As expected, the presence of BFA had marginal effect on the influence of Kif5bDN on the current density of Kv1.5. In addition, in a tetracycline-inducible expression system, the authors demonstrate that induced expression of Kv1.5 subsequent to expression of Kif5bDN significantly diminishes the magnitude of currents generated by Kv1.5 channels, presumably due to a block of channel delivery to the plasma membrane. Peculiarly, however, when investigated at steady-state level, coexpression of Kif5bDN and Kv1.5 resulted in a significant increase in the function of Kv1.5 channels. Zadeh et al. (2009) argue that the increase in surface expression of Kv1.5 upon coexpression of Kif5bDN is mediated through a distinct mechanism unrelated to that observed with coexpression of Kif5b. A previous study by the same group has demonstrated that inhibition of dynein increases the surface expression of Kv1.5 (Choi et al. 2005), prompting the authors to suggest that the increase in the current density of Kv1.5 at steady-state level by Kif5bDN is mediated by the inhibition of dynein function. Indeed, Zadeh et al. (2009) show that several manipulations that reduce channel internalization, potentially by interfering with dynein function, diminish the effect of Kif5bDN on the functional expression of Kv1.5. The effect of Kif5b, however, persisted even after inhibiting channel internalization.
Zadeh et al. (2009) have concluded that Kif5b is essential for the delivery of Kv1.5 channels to the plasma membrane in cardiac cells, which is perhaps the most likely and the simplest conclusion that can be drawn from their data. Several issues, however, remain to be addressed and some important experiments are needed to strengthen the conclusion of this study. In particular, the authors clearly demonstrate a dramatic increase in current density in two different cell lines upon coexpression of Kv1.5 and Kif5b. However, as evident from their data, the cell lines that they use express endogenous channels, the traffic of which may also be influenced by Kif5b. Therefore the contribution of Kv1.5 channels to the increase in current density observed upon expression of Kif5b is unclear. Since the endogenous channels appear to have similar kinetics to Kv1.5, it is particularly important to subtract their contribution from electrophysiological measurements in order to accurately and quantitatively estimate changes in Kv1.5 function. Furthermore, the authors note that they have been unable to demonstrate a direct interaction between Kv1.5 and Kif5b. It is therefore possible that the increase in current density associated with overexpression of Kif5b is due to increased surface expression of a ubiquitously expressed putative auxiliary subunit required for the function of Kv1.5. Thus, it is critical to quantitatively and directly measure the surface expression of exogenously expressed Kv1.5 channels before and after overexpression of Kif5b. Similarly, it is equally important to define the specificity of the effect of Kif5b on cardiac ion channel trafficking. Are Kv1.5 channels unique in their dependence on Kif5b or do other cardiac channels also require Kif5b for their trafficking to the plasma membrane? Is KLC a player in the delivery of Kv1.5 channels to the plasma membrane? What is the amino acid sequence in Kv1.5 that is required for enhanced functional expression by Kif5b and is that sequence conserved in any other voltage-gated potassium channel? Answers to these questions, amongst others, are central to solidifying the implication of kinesin-1 in the delivery of ion channels to the plasma membrane of cardiac cells. In addition, these answers may help in identifying novel therapeutic targets for cardiac disorders arising from deficient delivery of ion channels to the plasma membrane.
Acknowledgments
I am grateful to the Susan G. Komen Foundation for their support throughout my postdoctoral fellowship.
References
- Choi WS, Khurana A, Mathur R, Vishwanath V, Steele DF, Fedida D. Kv1.5 surface expression is modulated by retrograde trafficking of newly endocytosed channels by the dynein motor. Circ Res. 2005;97:363–371. doi: 10.1161/01.RES.0000179535.06458.f8. [DOI] [PubMed] [Google Scholar]
- Hirokawa N, Noda Y. Intracellular transport and kinesin superfamily proteins, KIFs: structure, function, and dynamics. Physiol Rev. 2008;88:1089–1118. doi: 10.1152/physrev.00023.2007. [DOI] [PubMed] [Google Scholar]
- Rivera J, Chu PJ, Lewis TL, Arnold DB. The role of Kif5b in axonal localization of Kv1 K+ channels. Eur J Neurosci. 2007;25:136–146. doi: 10.1111/j.1460-9568.2006.05277.x. [DOI] [PubMed] [Google Scholar]
- Zadeh AD, Cheng Y, Xu H, Wong N, Wang Z, Goonsaekara C, Steele DF, Fedida D. Kif5b is an essential forward trafficking motor for the Kv1.5 cardiac potassium channel. J Physiol. 2009;587:4565–4574. doi: 10.1113/jphysiol.2009.178442. [DOI] [PMC free article] [PubMed] [Google Scholar]